![]()
METHYLMERCURY
CYSTEINATE
How mercury reaches the human brain.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month April 2021
Also available: HTML version.
![]()

METHYLMERCURY
|
 |
Sometimes from fish...but we'll talk about that later.
Because this is a compound that can get into your body - and can end up in your brain - after you’ve been exposed to mercury.
Yes and No.
Let me finish. The shape of the molecule could be important to how it moves in the body, but two-coordination of mercury is not that unusual. A lot of mercury compounds, especially methyl-mercury compounds, have this kind of two-coordinate structure.
Apart from this compound, there are lots. Dimethyl mercury (MOTM October 2003) is a very simple example, this has a linear structure with a mercury-carbon bond length of 2.083 Å. Alkylmercury thiolates, such as CH3HgSPh, adopt it; the vaccine preservative Thiomersal (sodium ethylmercury thiosalicylate, MOTM February 2015) is a special example of this. This has a Hg-C bond of 2.07 Å, the same as CH3HgSPh.
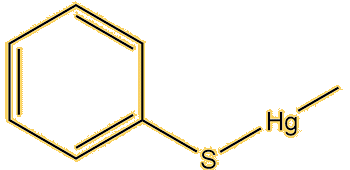 |
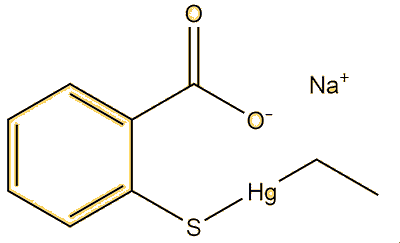 |
|
| Dimethylmercury | Methylmercury-phenylthiolate | Thiomersal |
Mercury has been getting into the environment for a very long while, but until the Industrial Revolution, it was at a much lower level. To measure it, scientists have taken samples from deep in the sediments of lakes or from ice in glaciers.
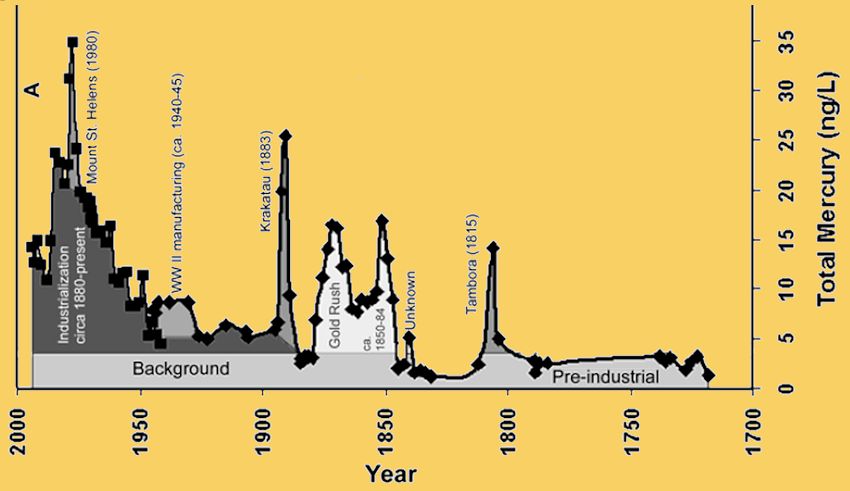
Atmospheric Mercury Deposition during the Last 270 Years.
Image: Public domain, taken from: P.F. Schuster et al., Atmospheric Mercury Deposition during the Last 270 Years:
A Glacial Ice Core Record of Natural and Anthropogenic Sources, Environ. Sci. Technol., 2002, 36, 2303-2310.
 The diagram above, depicting variations in mercury levels in the Upper Fremont Glacier, Wyoming, USA, shows the rise during the Industrial Age, from the time of American gold rushes, and also the spikes coinciding with volcanic eruptions into the atmosphere.
The diagram above, depicting variations in mercury levels in the Upper Fremont Glacier, Wyoming, USA, shows the rise during the Industrial Age, from the time of American gold rushes, and also the spikes coinciding with volcanic eruptions into the atmosphere.
Hundreds of years ago, alchemists were likely to get mercury poisoning from inhaling mercury vapour while trying to transmute mercury into gold. King Charles II of England (pictured, right) is thought to have been one of those poisoned.
During the later Roman Empire, people knew that mercury would amalgamate with metals like gold, and used that as a way of gilding copper based objects. In the late Middle Ages, mercury amalgamation started to be used as a way of extracting silver and gold from their ores, especially by the Spaniards in Mexico and South America. A lot of mercury escaped when the amalgams were heated to release the precious metals. This still happens today in gold-mining areas like the Amazon.

An area of the Peruvian Amazon that has been devastated by illegal gold mining.
The miners leave behind toxic pools contaminated with mercury which kills the plants
and animals for a wide area, as well as leaking into the water supply for nearby towns.
But everyone is exposed to mercury?Yes, but some more than others. In the mid-20th century agricultural workers and their families in countries like Iraq were poisoned when methylmercury compounds were used on seed grain as fungicides. This grain was intended only for planting, not for eating. But sometimes the farmers either did not understand – or ignored – the warnings on the sacks, and used the grain to make bread instead of planting it. This killed at least 459 people, although the actual death toll was maybe 10 times higher. Sometimes the grain was consumed by birds or small mammals, who in their turn went into the food chain of predatory birds. This led to the poisoning of large birds like owls and eagles. Even more serious was the discovery of mercury poisoning at Minamata in Japan. What happened?In the 1950s, people around Minamata in Japan observed strange behaviour and deaths in fish, birds, and animals like cats. Soon symptoms spread to inhabitants, and people started dying. This was traced to the Chisso chemical company's factory, which was using a mercury-containing catalyst to make acetaldehyde (ethanal) and discharging wastes into the Minamata river and later directly into Minamata Bay. By 2005, the number of certified cases of mercury poisoning (Minamata disease) in the area had risen to 2955 cases, with around 700 still alive then. |
 An Iraqi grain sack from 1971. The grain is coloured pink as a warning not eat it, but the warning labels on the sack are in Spanish which the Iraqi farmers could not read. Image: Wikimedia Commons. |
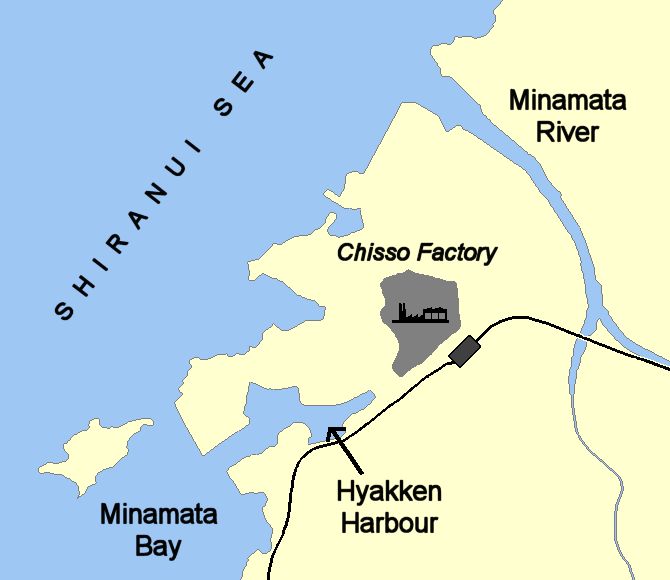 Map of Minamata bay illustrating the Chisso factory effluent routes. Image: Bobo12345 / CC BY-SA |
So this was mercury metal being released?No, it had been found that methylmercury ions, CH3Hg(aq)+, were being discharged from the plant. Later on it was found that 'inorganic' mercury could be methylated by bacteria using methylcobalamin in the environment, also being turned into methylmercury ions. |
Apart from gold miners and cases like that, many absorb it from releases into the environment from the burning of coal - which contains small amounts of mercury - by some coal-burning power stations, notably in the USA. And of course, fish eaters or eaters of other seafood.

Levels of mercury in commonly eaten fish. [Poster issued by the US Bureau of Health]
Some are more at risk than others. Methylmercury ions are taken up by small organisms like plankton, then eaten by small fish and eventually by large predators like swordfish and sharks, pike and some tuna and bass. These larger fish are long lived, therefore the methylmercury accumulates over the lifetime of the fish and can reach high concentrations, as they are not excreted. So if you eat shellfish, you are likely to be at much lower risk than if you eat pike, swordfish or shark. Among the victims are people living downstream of Amazonian gold-mining operations, who eat fish from the rivers.
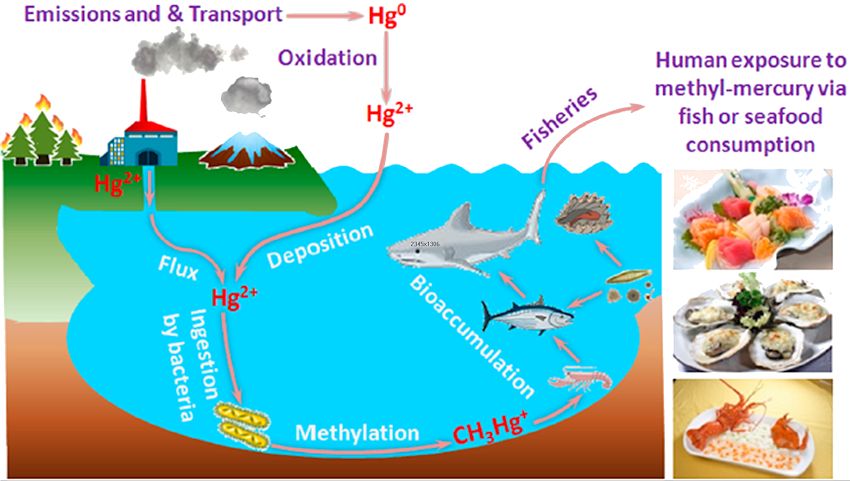
Bioaccumulation in the environment.
Reprinted with permission from L. Deng, Y. Li, X. Yan, et al., Anal. Chem. 2015, 87, 2452−2458.
Copyright 2015 American Chemical Society.
X-ray scattering spectra (XAS) can be used to measure bond lengths in compounds and distinguish between different types of mercury compounds. They were used to study mercury in two fish, swordfish and orange roughy. The spectra were identical to each other, showing that the same mercury compound was present in both fish, and also virtually identical to a two-coordinate methylmercury thiolate, most likely to be methylmercury(cysteinate), rather than other mercury compounds.
Yes, study of the XAS spectra of human hairs, taken from people in the Seychelles known to have mercury poisoning, have shown the same compound to be present. EXAFS (Extended X-ray Absorption Fine Structure) spectra indicated it has bond lengths of Hg-C of 2.06 Å and Hg-S 2.36 Å, very similar to the values known for CH3Hg(cysteinate) and CH3Hg(SPh). Chemical study showed that 80% of the mercury in the hair was present as this compound. XAS and EXAFS examination of the brain of an American professor who died of dimethylmercury poisoning showed that the dominant mercury species present was CH3Hg(cysteinate).
This means the mercury has bonded to cysteine, which is a common amino-acid present in the body It is found in high-protein foods and is actually a food additive with European E-number E-920. The sulfhydryl (SH) group has a high affinity for heavy metals, so that proteins containing cysteine will bind metal such as copper, zinc and iron, which are crucial for many metabolic processes - but they will also bind other metals such as cadmium, lead and mercury, which can be disastrous for the body.
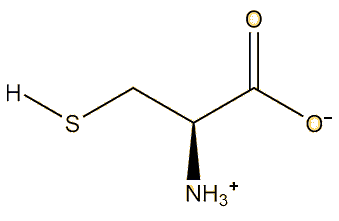 |
|
| Cysteine |
It spreads out in the body, attaining a steady-state concentration within two hours, and reaching the brain relatively quickly. It is disputed, but one theory is that a transporter that takes amino acids into the brain mistakes it for the amino acid methionine, because they have similar shapes, it is called ‘molecular mimicry’.
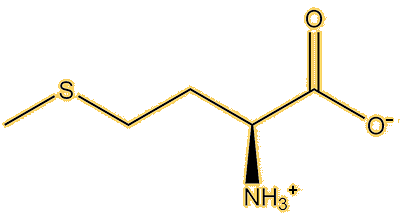 |
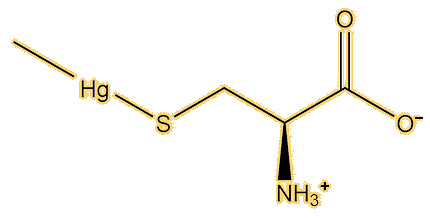 |
| Methionine | Methylmercury(L-cysteine) |
There is particular concern about its effect on cognitive and intellectual development, through the exposure of the foetus in the womb. Estimates from 2006 suggest that prenatal exposure to methylmercury causes cognitive impairment in over 300,000 children born in the U.S.A. each year, with an estimated 1,566 cases of mental retardation each year. Sobering statistics.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12357824]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12357824]