|

MPPP
1-methyl-4-phenyl-4-propionoxypiperidine,
or Desmethylprodine or ‘reversed meperidine’
The first ‘designer drug’ disaster

Simon Cotton
University of Birmingham

Molecule of the Month August 2023
Also available: JSMol version.

| 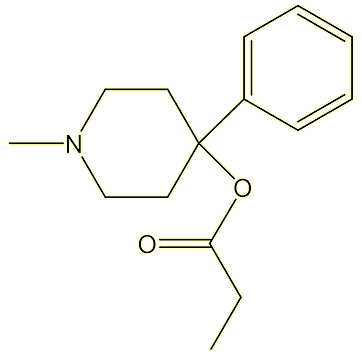 |
 What is a designer drug?
What is a designer drug?
The origin of the term is credited to Dr Gary L. Henderson, of the University of California at Davis. A designer drug is based on the structure of an existing drug – which may be naturally sourced from a plant (like cocaine or morphine) or be synthetic (like amphetamine) - but with a slightly different structure (often done by changing a side-chain) designed to make it undetectable in routine drug tests. These slight changes to its structure also change the properties of the drug in the body, sometimes dangerously. Thus, recently this has meant the ‘bath salts’ such as mephedrone in place of cathinone (khat), with amphetamine-like effects, or the ‘spice’ drugs, which affect the ‘cannabinoid’ receptor and may produce similar symptoms to marijuana.
So how long has this ‘designer drug’ been around?
What we call MPPP was first reported in the Journal of Organic Chemistry in 1947 by Albert Ziering and John Lee of the pharmaceutical company Hofmann-La Roche. They were trying to make better painkillers, though MPPP turned out to be no better than existing ones. They tested MPPP on lab rats and found that it seemed to be safe. To make it, you first react 1-methyl-4-piperidone with phenyllithium, then esterify the resulting alcohol with propionic anhydride.
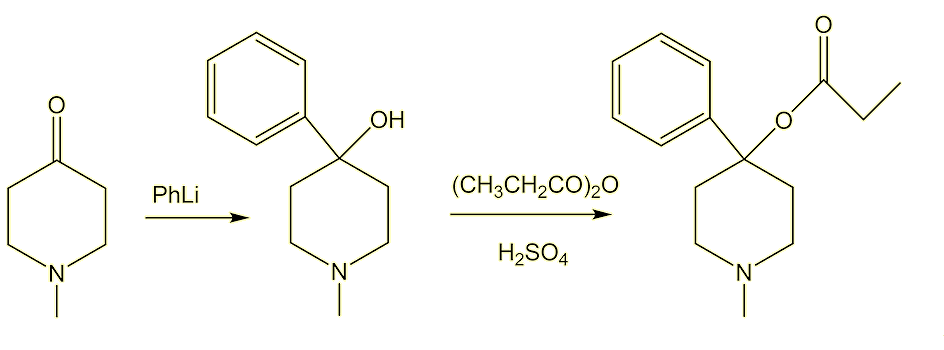
Why is it called reversed meperidine?
In comparison with the meperidine (a.k.a. pethidine or Demerol, the first synthetic opioid painkiller, originally reported in 1939), the ester group in MPPP is the other way round – it is an isomer of meperidine.
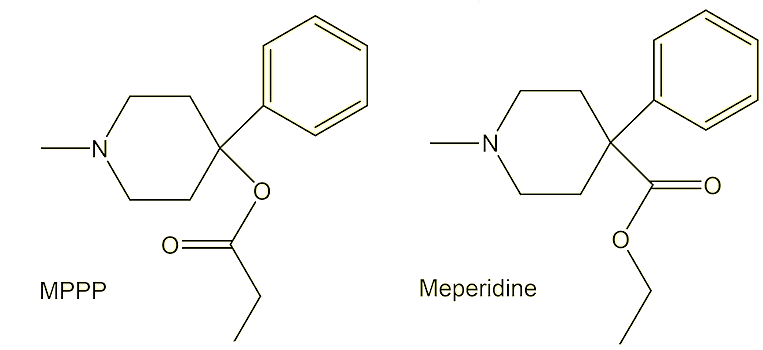
So who did find out the nasty side to the molecule?
A young chemistry graduate named Barry Kidston suddenly developed Parkinson’s disease in 1976, when he was only 23. For nearly a decade, he had been abusing a wide range of drugs, such as marijuana, amphetamines and barbiturates, before finally focussing upon opiates like meperidine and codeine. During the summer of 1976 he discovered the 1947 report of MPPP and decided to try that, successfully making it and testing it on himself, finding that it had an opiate-like ‘high’. He then repeated this synthesis several times. Whether he got over-confident in his ability or not, he seems to have taken some shortcuts in the synthesis and in 1976 developed a state of ‘muteness, severe rigidity, weakness, tremor, flat facial expression, and altered sensorium’, being unable to speak. He was admitted to a psychiatric ward.
So what happened next?
A number of treatments were tried. After treatments with a number of drugs including levodopa (a drug which the body converts into dopamine, used to treat Parkinson’s disease), his condition improved, and he received a number of medications until in September 1978 he was found dead from an overdose of cocaine and codeine, having continued on a path of drug abuse. Post-mortem examination of the brain revealed major destruction of nerve cells in the substantia nigra region, something also observed with Parkinson’s patients. They examined his laboratory in 1976 and found not just the MPPP but also an impurity formed at high temperatures, MPTP. This was tested on rats (and also on hamsters and guinea pigs), but without revealing any Parkinson’s symptom, so the cause remained a mystery. The medics studying the case wrote it up for publication in a journal called Psychiatry Research, and the medical world largely ignored it for a few years.
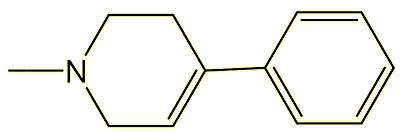
MPTP

Dr J. William Langston
[Photo: J. Parkinson's Dis.] |
So what changed?
In 1982, Dr J. William Langston, the Director of Neurology of the Santa Clara Valley Medical Centre in San José, was called in to examine George Carillo, a 42-year-old heroin addict who had just been admitted into the centre’s locked psychiatry unit. Carillo was ‘frozen’ – he could not move or talk. They spotted limited finger movement and after a while he was able to write very slowly: ‘I’m not sure what is happening to me. I only know I can’t function normally. I can’t move right.’ These symptoms of Parkinson’s disease had suddenly come on, over just a few weeks. And then he said he’d been taking heroin. The man’s 30-year-old girlfriend Juanita had the same symptoms. This puzzled Langston. People in their 30s and 40s were not supposed to get Parkinson’s. Certainly not from taking heroin. Then Langston found out about Bill and David, a young pair of brothers some 30 miles away in Santa Cruz, who had taken a ‘synthetic heroin’ and displayed similar ‘frozen’ symptoms. Through publicising these four cases, they learned of a drug dealer named Toby and his 21-year-old girlfriend Connie, with exactly the same history. At that point, someone recalled the article that had appeared in Psychiatry Research. |

George Carillo - a 'frozen addict' |
And?
Samples of the drugs which they had taken were sent for analysis, and found that what was supposed to be MPPP was largely made up of the impurity MPTP. Animal testing was extended to monkeys, and it was found that MPTP selectively kills cells in the substantia nigra, an area of the brain important in areas like motor control and learning, in rhesus and squirrel monkeys, just as in humans, so it affects the brains of primates in a different way to other animals.
Eventually George, Juanita and Connie were able to receive an experimental surgery in Sweden, during which they underwent transplanting substantia nigra cells from aborted foetuses into the damaged parts of their brains, and this helped them recover much of their motor function.
How had this happened?
Kidston seemed to have taken some short cuts in his synthesis in 1976, as had the West-Coast entrepreneur in 1982. If you try to carry out the esterification to make MPPP at too high a temperature, or too low a pH, an elimination reaction occurs. When Langston followed up the original 1947 synthesis of MPPP by going to the library of Stanford University, he found that the paper by Ziering and Lee had been cut out from the volume of Journal of Organic Chemistry with a razor blade. Obviously the chemist making this ‘new heroin’ did not want others in on the game.
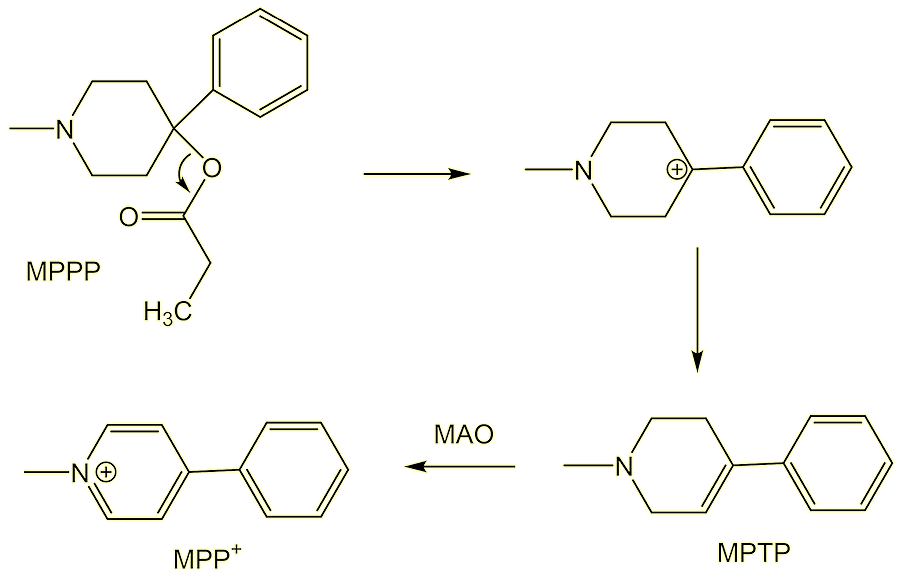
MPPP contains a tertiary carbon, which readily eliminates a carboxylate (a good ‘leaving group’) to generate a tertiary carbocation; this in turn loses H+ and is converted into 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP can cross the blood-brain barrier and, once in the brain, is mistaken for dopamine by monoamine oxidase (MAO) enzymes. Thus MPTP undergoes oxidation, forming MPP+, the species which is responsible for the neuronal damage.
Did any good come from this?
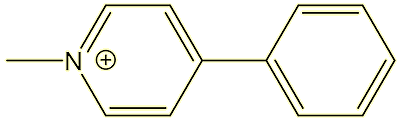
It has led to some new understandings about possible causes of Parkinson’s disease. For one thing, people spotted that MPP+ had a similar structure to the herbicide Paraquat, and scientists are working on a link between Parkinson’s and herbicide use.
 |
 |
| MPP+ |
Paraquat |
But - above all - the story of MPPP is another warning, a horrible one, about the dangers of dabbling in the wrong sort of chemistry.

Bibliography
- A. Ziering and J. Lee, J. Org. Chem., 1947, 12, 911-914 (synthesis of MPPP).
- G. C. Davis, A. C. Williams, S. P. Markey, M. H. Ebert, E. D. Caine, C. M. Reichert and I. J. Kopin,
Psychiatry Research, 1979, 1, 249-254 (Barry Kidston diagnosed).
- J. W. Langston, P. Ballard, J. W. Tetrud and I. Irwin, Science, 1983,
219, 979-980 (chronic Parkinsonism in humans due to a product of meperidine-analogue synthesis).
- R. S. Burns, C. C. Chieh, S. P. Markey, M. H., Ebert, D. M. Jacobowitz and I. J. Kopin,
Proc. Natl. Acad. Sci. USA, 1983, 80, 4546-4550 (testing MPTP in rhesus monkeys).
- J. W. Langston, I Irwin, E. B. Langston and L. S. Forno, Science, 1984,
225, 1480-1482 (pargyline prevents MPTP-induced Parkinsonism in primates).
- J. W. Langston, I. Irwin, E. B. Langston and L. S. Forno, Neurosci. Lett., 1984,
48, 87-92. (MPP+ as a toxin selective to the substantia nigra).
- J. N. Johannessen and S. P. Markey, Drug and Alcohol Dependence, 1984,
13, 367-374 (analysis of MPTP/MPPP mixture).
- P. A. Ballard, J. W. Tetrud and J. W. Langston, Neurology, 1985, 35, 949-956. (human Parkinsonism due to MPTP).
- R. M. Baum, Chem. Eng. News, 1985, 63, 7–16 (designer drugs)
- I. J. Kopin, Environmental Health Perspectives, 1987, 75, 45-51 (MPTP and Parkinson’s disease).
- G. L. Henderson, J. Forensic Sci., 1988, 33, 569-575 (designer drugs)
- H. Widner, J. Tetrud, S. Rehncrona, B. Snow, P. Brundin, B. Gustavii, A. Björklund, O. Lindvall and J. W. Langston, N. Engl. J. Med., 1992, 327, 1556-1563 (transplantation of fetal dopaminergic neurons as a treatment for MPTP-induced Parkinsonism).
- D. M. Perrine, The Chemistry of Mind-Altering Drugs, Washington DC, American Chemical Society, 1996, pp 78-81. (MPPP and the MPTP story).
- J. W. Langston and J. Palfreman, The case of the frozen addicts: how the solution of an extraordinary medical mystery spawned a revolution in the understanding and treatment of Parkinson's disease, New York, Pantheon, 1996.
- C. M. Tanner, F. Kamel et al., Environ. Health Perspect., 2011, 119, 866-872 (Rotenone, Paraquat, and Parkinson’s Disease).
- F. Kamel, Science, 2013, 341, 722-723 (herbicide-Parkinson’s link).
- J. W. Langston, Journal of Parkinson’s Disease, 2017, 7, S11–S22 (MPPP and the MPTP story)
- C. Vaccari, R. El Dib. and J. L. V. de Camargo, Systematic Reviews, 2017, 6, 98 (Paraquat and Parkinson’s disease: a systematic review protocol).
- How tainted drugs revolutionized Parkinsons research
- Profile of Dr William Langston
- Parkinsons disease and pesticides - what's the connection?


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.22013048]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.22013048]
![]()
![]()
![]()
![]()

 What is a designer drug?
What is a designer drug?






