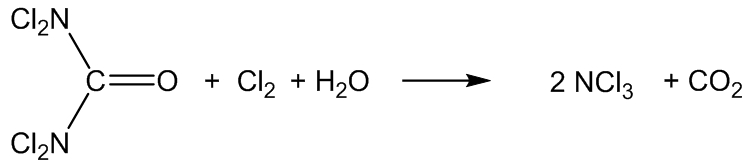Is it anything like nitrogen triiodide?
It most certainly is, it has a similar structure to NI3 (MOTM Dec 2001) and is even more unpredictably explosive.
 So it is a molecule that’s only been recently studied?
So it is a molecule that’s only been recently studied?
Not at all, it was first made in 1812 by the Frenchman Pierre Louis Dulong (1785 –1838), whose portrait is shown on the right.
Never heard of him.
Along with Alexis Thérèse Petit (1791-1820), he is probably most famous for Dulong and Petit’s law, which gives a formula for the specific heat capacity c of a solid material:
c = 3R / M
where R is the gas constant and M is the molecular mass. This applies to many substances.
What’s that got to do with NCl3?
Absolutely nothing at all, but it shows the wide range of topics that experimental scientists could embrace 200 years ago. Anyway, back to NCl3. Dulong made it by the reaction of chlorine gas and ammonium chloride solution.
NH4Cl + 3 Cl2 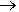 NCl3 + 4 HCl
NCl3 + 4 HCl
As the discoverer, he was not to know that this oily yellow liquid was violently explosive, and it cost him an eye and two fingers (accounts vary as to how many fingers). Dulong persisted in his researches and discovered that it boiled at around 70°C.
So Dulong spread the word about its dangers?
He told Sir Humphrey Davy about his findings. Showing true scientific curiosity, Davy repeated the synthesis (and the explosion); a piece of glass lodged in his cornea. It was while he was temporarily blinded that Davy hired Michael Faraday as his assistant. Faraday also experienced the explosive power of NCl3. He wrote:
I was able to escape four violent explosions. The most terrible of them happened when I held between my thumb and index finger a small test-tube containing seven grains of nitrogen trichloride. The explosion happened so suddenly that on opening my hand some of my nail was torn away; my fingers were burnt so much that I still am unable to use them like before.

Sir Humprey Davy as he might have
appeared after his accident. |

Michael Faraday after experiencing
the explosive power of NCl3. |
In a chemistry book of 1857 we can read:
The chloride of nitrogen is the most violently explosive substance yet discovered, and should not be experimented upon by the student in quantities larger than a mustard seed at a time, and even in this quantity, with great caution. Both its discoverer (Dulong), and Sir H. Davy, notwithstanding their experience and caution as chemical experimenters, were seriously injured by its violence.
How do people make NCl3 nowadays?
When they have to, they employ the electrolysis of slightly acidic ammonium chloride; a current of air is passed through the mixture so that the NCl3 is removed as fast it is formed (it can also be extracted in non-polar solvents like CCl4). A mixture of air with NCl3 is much more stable than pure NCl3. Others use the reaction of a mixture of Cl2 and N2 diluant with (NH4)2SO4. Best not attempted.
Why is NCl3 so dangerous?
The danger is that its sparing solubility in water causes explosive liquid drops of NCl3 to be formed (metallic surfaces also catalyse its decomposition). NCl3 isn’t just an explosive hazard though; its toxicity causes concerns for workers at indoor swimming pools, such as attendants and lifeguards. Because it is insoluble and volatile, NCl3 readily escapes into the air above a pool, and is a particular hazard in indoor swimming pools.

The air near an indoor swimming pool can contain NCl3.
How does it get into swimming pools?
It is also formed in swimming pools by the reaction of Cl2 disinfectant with nitrogen compounds, especially urea ((H2N)2C=O), amino acids, creatinine and ammonium salts.
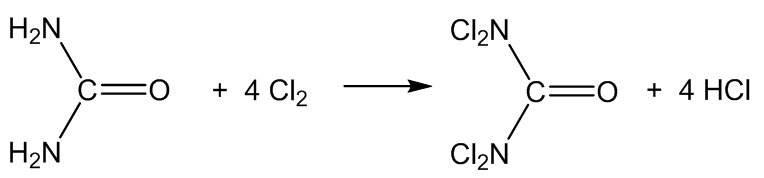
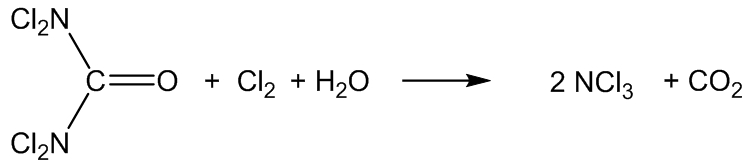
The urea comes from urine, I suppose?
Yes, but also from the skin – urea is a degradation product of the amino acid arginine in the skin.
Does it have a smell?
Its smell is very similar to chlorine, but the odour threshold is much lower, 0.02 mg/l compared to 20 mg/l. When you smell the air at a swimming pool, you may well be detecting NCl3.
And it is a hazard?
Along with the other chloroamines, monochloramine (NH2Cl) and dichloramine (NHCl2), it is a powerful irritant, with a toxicity similar to Cl2, and has been linked with irritation to the eye and upper airway, as well as causing asthma. A 2007 Dutch study showed that swimming pool workers showed a higher level of airways symptoms in comparison with the population in general, whilst poolside workers showed more frequently work-related upper respiratory symptoms compared with administrative staff.
So presumably it is difficult to study NCl3?
Yes, but quite a bit is known about it. It melts at -40°C (233 K) and boils at 71°C (344 K), and is scarcely soluble in water.
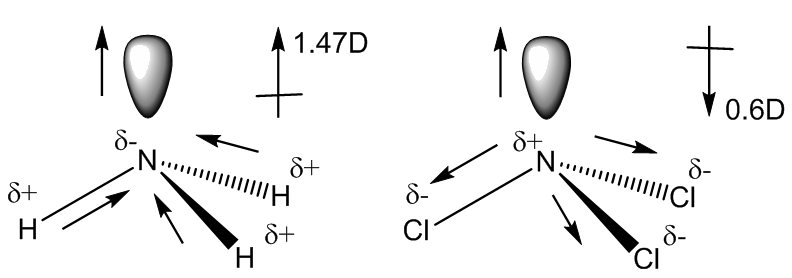
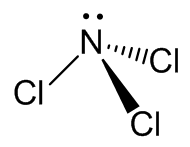
As expected, the molecule has a pyramidal structure with C3v symmetry, like NH3.
| | X-ray diffraction (solid) | Electron diffraction (vapour) | Microwave (vapour) |
| N-Cl (Å) | 1.75 (0.01) | 1.759 (0.002) | 1.7535 (0.002) |
| angle Cl-N-Cl (°) | 106.8 (2) | 107.1 (0.5) | 107.8 (0.33) |
It has a dipole moment of 0.6 Debye, numerically smaller than that of ammonia (1.47 Debye). The reason for this is that in ammonia the polarities of the lone pair and the N-H bonds operate in the same direction, whilst in NCl3 (and NF3), because the halogen is more electronegative than nitrogen, the bonds are polarised the other way round. Therefore in NCl3 the polarities of the bonds and of the lone pair operate in opposite directions and to some extent cancel out.
NCl3 is not very basic and is decomposed by strong acids.
NCl3 + 4 HCl  NH4Cl + 3 Cl2
NH4Cl + 3 Cl2
Why is NCl3 so unstable?
The enthalpy of formation of NCl3 is + 232 kJ mol-1, a fairly large endothermic value, a good sign that a compound is unstable. This refers to the process:
½ N2(g) + 3/2 Cl2(g)  NCl3(l).
NCl3(l).
This means that the enthalpy change for the reverse reaction, the decomposition of NCl3, is pretty exothermic.
NCl3(l)  ½ N2(g) + 3/2 Cl2(g)
½ N2(g) + 3/2 Cl2(g)
The positive entropy change associated with the formation of the gaseous chlorine and nitrogen would also favour the decomposition, as would the very high N-N bond energy (~940 kJ mol-1).

Bibliography
- Chapman and Hall Combined Chemical Dictionary compound code number: - HTZ52-L
- W. E. Dasent, Nonexistent Compounds, Edward Arnold, London, 1965, pp 30-31 (stability of NX3)
- P. Kovacic, M. K. Lowery and K. W. Field, Chem. Rev., 1970, 70, 639–665. (Chemistry of N-Bromamines and N-Chloramines)
- L. Bayersdorfer, U. Engelhardt, J. Fischer, K. Höhne and J. Jander, Z. Anorg. Allg. Chem, 1969, 366, 169–179 (IR and Raman spectrum).
- H.B. Bürgi, D. Stedman and L.S. Bartell, J. Mol. Struct., 1971, 10, 31-38 (electron diffraction)
- G. Cazzoli, P. G. Favero and A. Dal Borgo, J. Mol. Spec., 1974, 50, 82–89. (microwave spectrum)
- H. Hartl, J. Schöner, J. Jander and H. Schulz, Z. Anorg. Allg. Chem., 1975, 413, 61–71. (crystal structure at −125°C)
- J. Jander, Adv. Inorg. Chem., 1976, 19, 1–63 (review of NCl3, NBr3 and NI3).
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth Heinemann, 2nd edition, 1997, pp 438-441
- P. Cardillo, J. Loss Prevent. Proc., 2001, 14, 69–76 (“Some historical accidental explosions”)
- H. H. Sisler, in Encyclopedia of Inorganic Chemistry, R.B. King ed, Wiley, 1994, Vol.5 pp 2545-2551.
- A.Hammerl and T.M. Klapötke, Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley, 2011. DOI: 10.1002/9781119951438.eibc0147
NCl3 and swimming pools
- K. M. Thickett, J.S. McCoach, J. M. Gerber, S. Sadhra and P. S. Burge, Eur. Respir. J., 2002, 19, 827-832.
- E. Stottmeister and K. Voigt, Archiv des Badewesens, 2006, 3, 158-162; Recreation, March 2006, 30-33.
- J. H. Jacobs, S. Spaan, G. B. G. J. van Rooy, C. Meliefste, V. A. C. Zaat, J. M. Rooyackers and D. Heederik, Eur. Respir. J., 2007, 29, 690–698
- J. Li and E. R. Blatchley, Environ. Sci. Technol., 2007, 41, 6732-6739
- S.-C. Weng, W. A. Weaver, M. Z. Afifi, T. N. Blatchley, J. S. Cramer, J. Chen and E. R. Blatchley, Indoor Air, 2011, 21, 391–399
- C. Schmalz, F. H. Frimmel and C. Zwiener, Water Research, 2011, 45, 2681 -2690.
- J. Parrat, G. Donze, C.Iseli, D. Perret, C. Tomicic and O. Schenk, Ann. Occup. Hyg., 2012, 56, 264-277.
- G. Fantuzzi, E. Righi, G. Predieri, P. Giacobazzi, B. Petra and G. Aggazzotti, J. Expo. Sci. Environ. Epidemiol., 2013, 23, 88–93.
- T.-S. Chu, S.-F. Cheng, G.-S. Wang and S.-W. Tsai, Sci. Total Environ., 2013, 461-462, 317-322
- M. Ostrowski and R. R. Brooks, Water Conditioning & Purification, August 2014, 56(8) 3pp
- “A Healthy Respect for Pool Chemicals Can Help Avoid Accidents and Injuries”.
- http://www.chloramine.org/articles_pdf/Chemicals_in_Drinking_Water_Chloramines.pdf (chloramines in drinking water)
- Celia Henry Arnaud, ‘What lies beneath’, Chem Eng. News, August 1st 2016, pp 28-32 (The chemistry of swimming pools, including NCl3)


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5260021]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5260021]

![]()
![]()
![]()
![]()
 So it is a molecule that’s only been recently studied?
So it is a molecule that’s only been recently studied? NCl3 + 4 HCl
NCl3 + 4 HCl