
Seville oranges
[Image: A. Barra - GFDL]
![]()
Neohesperidin
The bitter taste in bitter-oranges
that can be converted into a sweetener.
![]()
![]()
Molecule of the Month November 2016
Also available: HTML version.
![]()
|
NeohesperidinThe bitter taste in bitter-oranges
|
Most of them are, but not all of them. The ones that grow all along the streets in Seville, Spain, are so-called bitter oranges, which are so bitter they cannot be eaten raw – and indeed, it is often dangerous to do so.
Seville oranges, their peel, and their extract, contain 3 compounds called N-methyltyramine, octopamine and synephrine, which have similar effects on the body to adrenaline, namely increasing blood pressure and heart rate. There are reports that bitter orange supplements have been linked to strokes, angina and even a heart attack!
It has been used in herbal medicine as a stimulant and appetite suppressant, although this is declining now due to the possible risks mentioned above. The essential oils from these oranges can be used in perfumes, as a flavouring or as a solvent. But the biggest use for Seville oranges is to make marmalade.
Paddington Bear’s favourite sandwich ingredient?Yes, that’s right. The juice and peel of Seville oranges are boiled with sugar and water. This causes pectin, a complex set of polysaccharides (sugars) present in the cell walls of the orange, to dissolve into the surrounding hot water. When the mixture cools the pectin then sets into a jelly-like consistency. And voilá, you have orange marmalade. How is that different from jam?There are two main differences. Jams are made in the same way, but usually not with citrus fruits. Marmalades are always made with citrus fruits, especially Seville oranges. Also, marmalades tend to have a bitter tang to them, whereas jams are purely sweet. |
 Paddington Bear eating marmalade sandwiches. |
In Seville oranges it is a molecule called neohesperidin which is not present in normal sweet oranges. The presence of tiny amounts of this molecule is enough to overwhelm the sweetness of the sugars that are also present and make the orange taste bitter.
|
|
But in a strange quirk of chemistry it is possible to convert bitter neohesperidin into a sweet-tasting molecule called Neohesperidin dihydrochalcone, or NHDC for short.
In the 1960s the US Department of Agriculture has a research project to find methods to reduce the bitterness in citrus fruits. The 2 chemists involved (R.M. Horowitz, R.M. & B. Gentili) found that if neohesperidin was reacted with a strong base like KOH it opened one of the rings in the molecule. Hydrogenation then yielded a new compound, NHDC, which was about 340 times sweeter than the equivalent weight of sugar. The new molecule was stable at high temperatures, so could be used as an artificial sweetening agent in hot drinks or cooking. Because of its origin, it’s often marketed as a ‘natural’ sweetener, a bit like stevioside [MOTM Sept 2005]. NHDC is approved in the EU and has an ‘E-number’ (E959), and in the Far East, but still hasn’t yet been approved for use in human foodstuff by the FDA in the US.
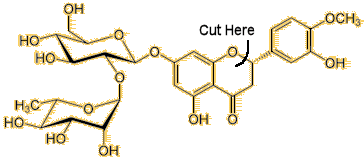 |
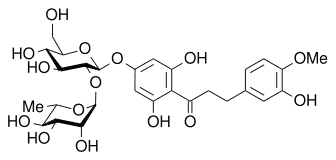 |
| Neohesperidin (Very bitter) | NHDC (Very sweet) |
 An advert for NHDC sold by a Chinese chemical company. |
It’s often used by pharmaceutical companies as an additive to reduce the bitterness of some drug tablets. It’s also sometimes found in animal foods (the sweet taste makes them eat their bland food faster), and as a flavour-enhancer in toothpaste, mouthwash, ketchup and mayonnaise. But for chemists, the most curious thing about NHDC and neohesperidin is that by changing just one functional group, a very bitter molecule can become a very sweet one. And this is beginning to give food scientists clues as to how humans perceive and differentiate different tastes.
![]()
![]()