![]()
Ninhydrin
It turns amino acids deep purple.
![]()
Li Liang
National Chung Hsing University, Taiwan
![]()
Molecule of the Month April 2018
Also available: JSMol version.
![]()

 |
NinhydrinIt turns amino acids deep purple.
Li Liang
Molecule of the Month April 2018
|
 |
No, the colour purple. And, before you say it, not the movie of that name either.
It’s the trademark of the chemical registered in 1912, derived from the German name of the chemical triketo hydrindene.
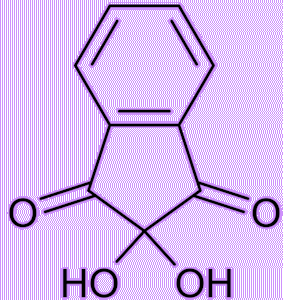
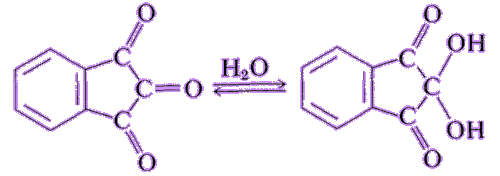
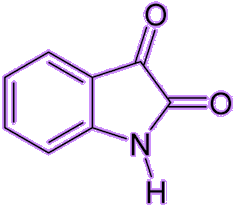
It’s a stable hydrated product of indane-1,2,3-trione. It is a white to light yellow solid that can dissolve in many solvents such as water, ethanol, and acetone at room temperature. Except for tertiary amines, it can react with ammonia, primary and secondary amines, and peptides, forming a beautiful purple product.
 |
 |
| Indane-1,2,3-trione Ninhydrin | White ninhydrin solid |
So?As a result, it is one of the most important and widely used reagents for detecting and analyzing amino acids, most of which are colourless. Why do you want to analyse amino acids?Amino acids are molecules with a carboxylic acid at one end and an amine group at the other. This means they can link together into chains via amide bonds. These polymeric chains, which we call proteins, can involve thousands of linked amino acids. In order to find out which amino acids make up a protein, the protein is chemically chopped up into smaller sections, and the pieces separated by chromatography. But in order to see the stripes in the chromatogram, they need to be reacted with ninhydrin to turn them purple. |
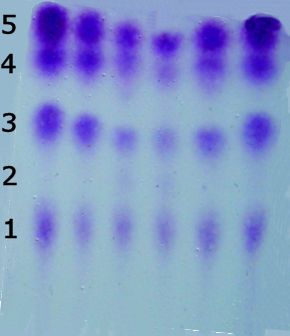 A chromatogram of a protein sample stained purple with ninhydrin, showing 4 amino acid pieces present at different positions and one missing (number 2) at its expected location. |
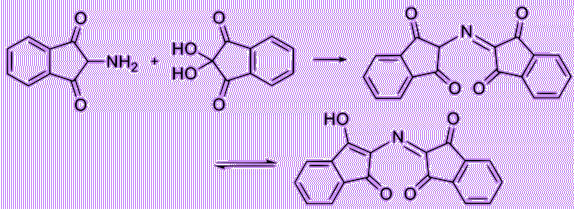
Let’s have a deeper look of the reaction of ninhydrin with alpha amino acids. First, ninhydrin is dehydrated and reacts with an amino acid, forming a Schiff base. Then, it undergoes decarboxylation, releasing a carbon dioxide. Finally, with its reaction with water, the bond with side chain (R group) then quickly departs from the imino intermediate, forming an aldehyde and diketohydrindamine.
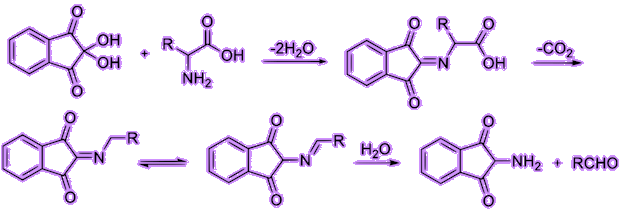
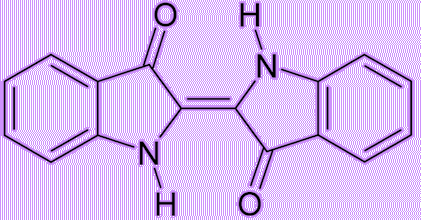
Because the intermediate diketohydrindamine is a primary amine, it condenses with ninhydrin again, forming the final dimeric imino derivative, which is often called Ruhemann’s purple.
 Who was Ruhemann?
Who was Ruhemann?Siegfried Ruhemann (photo, right) was a chemist who is virtually unknown to the chemical community today. In 1910, at the age of 51, he accidentally discovered ninhydrin when he was a University Lecturer in Chemistry at Cambridge. A few months later, he also observed the colour reaction of ninhydrin with ammonia and amino acids, noting: "of especial interest is the action of ammonia on the triketone. If the aqueous solution of the mixture of both substances is kept for a short time it turns a deep reddish-violet." As he was about to work further on this topic, increasing anti-German hysteria in Britain since the outbreak of World War I forced him to resign his lectureship in Cambridge and eventually return to Germany after the war, where he had obtained most of his education. He then worked in Emil Fisher’s laboratory for some years and in 1921 found a position as head of a small industrial research institute, working on lignite and peat chemistry.
In the late nineteenth century, many chemists worked hard to explore and even emulate the colourful world by investigating and reproducing natural dyes. At that time in Europe, dyestuffs, mostly imported from Asia, were tremendously expensive and only a few had ever been used on a commercial scale. Among various colours of dyes, purple was one the most valued and coveted one because of its scarcity.

After the serendipitous discovery of the synthesis of mauveine (MOTM January 1996) by chemist William Henry Perkin in 1856, many scientists recognized the importance of synthetic dyes. They were even playing a part in the politics of the time (see MOTM Jan 2016 on Congo Red).
 |
| Mauveine B |
In 1878, Adolf von Baeyer synthesized the blue-coloured indigotin (MOTM February 2009) from isatin, and later received the Nobel Prize in Chemistry 1905 for his work on organic dyes and hydroaromatic compounds.
 |
 |
| Indigotin | Isatin |
Ruhemann’s purple, a molecular structure resembling that of indigotin, might have been a potentially feasible dye. However, ninhydrin was so expensive that it was impractical to use it as the starting material in the mass production of the purple dye.
Although it’s impractical to implement ninhydrin for the use of dye industry, the colour reaction of ninhydrin is actually extremely useful in a broad spectrum of disciplines such as biochemistry, food industry, microbiology, histochemistry, and protein science. In addition to the common application of ninhydrin as a reagent to locate amino acids on chromatograms, many techniques and improvements of the ninhydrin reaction have been created to detect and analyze various chemical compounds, tissues, foods, and drugs of interest.
Ninhydrin is also an essential reagent used in the field of forensic science.
 Like in TV crime series?
Like in TV crime series?Yes, ninhydrin has been extensively used in the visualization of latent fingerprints. If you've ever watched TV shows such as CSI (Miami, NY, etc) or Silent Witness the forensic scientists will often refer to ninhydrin when they're swabbing for fingerprints. In 1913, a few years after the discovery of ninhydrin, scientists Emil Abderhalden and H.S. Schmidt commented: "Sweat contain substances which dialyze and react with ninhydrin. The fact that sweat gives an intense reaction is of importance in carrying out the test. Caution must be taken that nothing is touched which later comes in contact with the reagent."
 In spite of the fact that ninhydrin reacting with sweat has been well-known since then, it was not until the 1950s that this application started to be employed in forensic science. Fingerprints are made from sweat, which mostly is water, with some salts, lipids, and amino acids, many of which can be used to develop fingerprints. For example, most lipids can be stained by iodine vapour, and salts can react with silver nitrate to form light-sensitive silver halide; and of course, ninhydrin can react with amino groups from fingerprints. While there are a dozen or so reagents that serve the purpose well, ninhydrin is especially useful because it can develop latent fingerprints on porous surfaces such as paper. In practice, ninhydrin is often dissolved in a volatile solvent such as acetone or ethanol and sprayed onto a surface. After reaction with the terminal amine groups in the amino acids and proteins in the fingerprint residue, Ruhemann’s purple is formed. Many ninhydrin analogues, with the same active cyclic vicinal triketone part, have been developed since the 1980s to enhance the colour and fluorescence of the original Ruhemann’s purple. However, ninhydrin is still the universal reagent in forensic science because most of the analogues are too expensive and thus are not commercially available.
In spite of the fact that ninhydrin reacting with sweat has been well-known since then, it was not until the 1950s that this application started to be employed in forensic science. Fingerprints are made from sweat, which mostly is water, with some salts, lipids, and amino acids, many of which can be used to develop fingerprints. For example, most lipids can be stained by iodine vapour, and salts can react with silver nitrate to form light-sensitive silver halide; and of course, ninhydrin can react with amino groups from fingerprints. While there are a dozen or so reagents that serve the purpose well, ninhydrin is especially useful because it can develop latent fingerprints on porous surfaces such as paper. In practice, ninhydrin is often dissolved in a volatile solvent such as acetone or ethanol and sprayed onto a surface. After reaction with the terminal amine groups in the amino acids and proteins in the fingerprint residue, Ruhemann’s purple is formed. Many ninhydrin analogues, with the same active cyclic vicinal triketone part, have been developed since the 1980s to enhance the colour and fluorescence of the original Ruhemann’s purple. However, ninhydrin is still the universal reagent in forensic science because most of the analogues are too expensive and thus are not commercially available.
Ninhydrin is an important starting material in organic synthesis. It is used in the design and synthesis of various frameworks, particularly in the preparation of heterocyclic compounds. Some of those compounds are potential anticancer agents. Ninhydrin is also a useful tool in geochronology, a science of determining the age of fossils.
When ninhydrin reacts with collagen from fossil bones, carbon dioxide gas is released. Because the carbon source of the carbon dioxide is obtained from the collagen, a conventional radiometric dating technique called gas proportional counting can then be applied to determine the age of the fossil by measuring the beta particles of radiocarbon decay.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5972428]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5972428]