![]()
![]()
![]()
Also available: HTML only, VRML and JMol versions.
![]()
Osmium tetroxide (right) is probably the best-known compound of Osmium, which can easily be found, as it is just two places below Iron in the Periodic Table. Osmium tetroxide is inextricably linked to the discovery and subsequent purification of Osmium. |
Osmium is a scarce metal found in only 0.005 ppm of the Earth's crust. Tennant discovered Osmium in 1803 in the residues left behind when crude Platinum and other precious metals had been dissolved in aqua regia (c.HNO3/c.HCl). It is named from the Greek word for odour (osme). Osmium is also found, often with Ru and Ir, in the anode sludges produced during the electrolysis of Nickel (II) solutions. Osmium has an exceptional density of 22.57 g cm-3 and a very high m.p. of 3045°C.
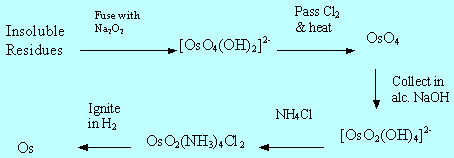
Flow diagram showing the extraction of osmium via OsO4
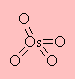 Osmium tetroxide, also known as osmium(VIII)oxide, formula OsO4, is an example of the highest oxidation achieved by a transition element. Like methane (CH4), osmium tetroxide has a tetrahedral geometry with the O-Os-O bond angle(s) approximating 109.5°. It is an extremely toxic crystalline solid with a low m.p = 40°C and bp = 130°C. The powerful combination of high toxicity and high volatility (easily evaporates) makes osmium tetroxide dangerous if not stored or handled correctly. Recent media exposure suggested its use as a 'billionaires' chemical weapon. This Molecule of the Month article is written to show how important this reagent is, to the chemical community, and how it was party to the awarding the Nobel Prize in Chemistry in 2001 to K. Barry Sharpless.
Osmium tetroxide, also known as osmium(VIII)oxide, formula OsO4, is an example of the highest oxidation achieved by a transition element. Like methane (CH4), osmium tetroxide has a tetrahedral geometry with the O-Os-O bond angle(s) approximating 109.5°. It is an extremely toxic crystalline solid with a low m.p = 40°C and bp = 130°C. The powerful combination of high toxicity and high volatility (easily evaporates) makes osmium tetroxide dangerous if not stored or handled correctly. Recent media exposure suggested its use as a 'billionaires' chemical weapon. This Molecule of the Month article is written to show how important this reagent is, to the chemical community, and how it was party to the awarding the Nobel Prize in Chemistry in 2001 to K. Barry Sharpless.

Osmium tetroxide is probably colourless (Butler and Harrod, Inorganic Chemistry: Principles and Applications, p.343) but is sometimes described as yellow (see photo above). I suggest this discrepancy lies with the presence of the impurity OsO2, which is yellow-brown in colour (Cotton and Wilkinson, Advanced Inorganic Chemistry, p.1002). It is very soluble in CCl4 (carbon tetrachloride = tetrachloromethane) and moderately soluble in water. OsO4 has a characteristic ozone-like odour. The smell is recognisable as the 'fresh' smell you get by old photocopiers, near someone welding, or to be sniffed at on a sunny summers day in a heavily congested city. Should you be close to a lightning strike you will smell ozone, and this is far more likely than being close to a terrorist atrocity.
Osmium tetroxide is particularly hazardous to the eyes as it is readily reduced by living tissue to give a black oxide and or the metal. For this reason it finds itself an important use as a Biological Stain when in dilute aqueous solutions.
OsO4 is a convenient reagent for the same side (syn) addition of two OH groups across a carbon-carbon double bond. Osmium tetroxide was first given this use back in 1936 by Criegee and a good source of information on this powerful oxidizing agent is to be found in Schroder, Chem. Rev. 80, 187-213 (1980).
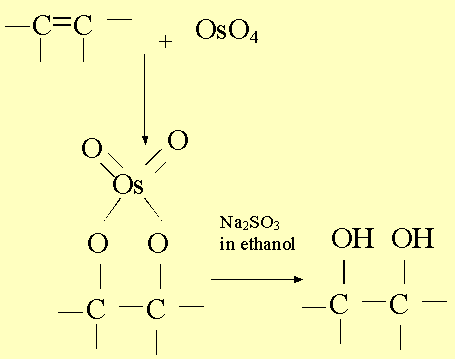
This is a rather slow addition but has the distinct advantage of being almost quantitative, an important consideration when performing multi-step syntheses of natural products.
A more familiar reagent that also adds two OH groups across a double bond is alkaline KMnO4. This is a reaction, which used to be taught at A-level and perhaps more interesting than the often-quoted 'decolourises bromine water' test for unsaturation.
The high cost of osmium tetroxide does not preclude its use by perennially under funded chemical research groups. Fortunately, the same reaction can be achieved catalytically. Either H2O2 with OsO4 (Milas & Sussman, J. Am. Chem. Soc., 58, 1302 (1936) or N-methylmorpholine-N-oxide with OsO4 (VanRheenan, Kelly and Cha, Tet. Lett, 1973 (1976). I also made use of this reagent during my own research with Professor Pettit, J. Chem. Soc. Chem. Commun. 1994, 2725-6.
Addition of oxygen (oxidation) is possible by burning the metal at 800°C.
Os + 2 O2 ![]() OsO4
OsO4
An alternative preparation is the oxidation of osmium salts with c.HNO3. This is a widely used method to recover osmium via the volatile osmium tetroxide.
OsO4 is a more powerful oxidizing agent than the halogens. It oxidizes the halides (Cl-(aq), Br-(aq), I-(aq)) to their respective halogens. One easy way to recognise oxidations is an increase in oxidation number (from 1- of the halides to 0 for the elemental halogens).
2 Cl- (aq) ![]() Cl2
Cl2
OsO4 + 2 OH- (aq) ![]() [OsO4(OH)2]2- [deep red]
[OsO4(OH)2]2- [deep red]
The above reaction is interesting for a number of reasons, i.e. surprising colour change, osmium expands its coordination sphere to six, the product is diamagnetic and the OH groups are trans (opposite each other).
Osmium tetroxide is a valuable tool in the research of both biologists and chemists, and needs to remain so. Chemists need to protect their interests, perhaps more overtly in future, and ensure that they continue with their important work against the tide of negative publicity concerning the misuse of chemicals by non-chemists.
![]()