
High Level Ozone:
Introduction
The majority of ozone in the earth's atmosphere is found in the stratosphere(15-50km above the earth's surface) This high level ozone plays a crucial role, protecting animals and plants from the suns harmful ultra violet rays, and stabilising the earth's climate. This ozone layer is incredibly unstable, since it is constantly being formed and broken down through interactions with UV radiation. But, as it is so reactive the ozone layer can easily be broken down by pollutant gases rising from the earth. CFC's and nitrous oxide are thought to be particularly responsible for the breakdown of the ozone layer. Since the hole in the ozone layer has developed cases of skin cancer, particularly have grown. Return to Top
The Role of the Ozone Layer
The primary role of the ozone layer is to prevent the sun's harmful ultra violet rays from reaching life on earth. This UV radiation is of a suitable energy to damage living cells, causing sunburn which can lead to skin cancer. The areas of the planet with thinner ozone coverage have much higher incidences of skin cancer. This is particularly true around the equator. It is thought that a reduction of 1% of the ozone in the stratosphere could lead to:
- An increase in skin cancers in animals and humans
- A suppression of the human immune systems
- Some inhibition to plant life, and an increased susceptibility to pests
- Reduction in growth of phytoplankton, endangering the food chain
- A decrease in aquatic lifeforms
Ozone absorbs UV radiation in its formation and breakdown: O2absorbs UV light to form 2 oxygen radicals:
O2 + hv  O + O (1)
O + O (1)
O+O2 O3 (2)
O3 (2)
O+O O2 (3)
O2 (3)
O+O3  2O2 (4)
2O2 (4)
O3 + hv  O2 + O (5)
O2 + O (5)
Ozone in the stratosphere also plays a role in absorbing some of the infra-red radiation which travels from the earth into space, keeping the earth warm. In this role it is partly responsible for global warming. The primary culprits for this, however, are the pollutant gases from human activities which seem to exaggerate the effect, making the earth too warm.

The Ozone Hole:
In the early 1980's scientists who had been studying the atmosphere above the Antarctic realised that ozone was disappearing from the area every spring. Data showed that the total amount of ozone in the atmosphere declined by 2% between 1978 and 1985. This may not seem much, but ozone is a trace element in the stratosphere maintaining a delicate balance. There are only a few parts of ozone per billion.
Why were ozone levels decreasing? The answer came when Chlorofluorocarbons, were found in the stratosphere. These chemicals had originally been developed because they were so stable. They were used as refrigerants in air conditioners and fridges, Blowing agents in expanded plastics(e.g. polystyrene), Aerosol Propellants, and Cleaning Solventsto dissolve grease, particularly in dry cleaning. The stability of these gases mean that they can exist in the atmosphere for as long as 75 years. During this time they can be carried by winds into the stratosphere. It is here where they breakdown, due to the sun's high energy radiation, into hydrogen fluoride and chlorine. It is the chlorine and the fluorine which are responsible for the destruction of the ozone layer. Nitrogen oxides are also thought to be partly responsible for the decay of the ozone layer, but they can also rebuild it as shown by the equations in Low Level Ozone.
The Breakdown of Ozone:
Here chlorine will be used as the example: When CFC's are destroyed they form Cl radicals. These chlorine radicals react with ozone:
Cl+O3 O2+ClO (6)
O2+ClO (6)
ClO+O Cl+O2 (7)
Cl+O2 (7)
O+O2 O3 (2)
O3 (2)
The hole in the ozone layer is particularly bad over Antarctica, although most of the CFC's are
released in the Northern Hemisphere. This is because winds blow the CFC's to the poles. Below is a
graph of the October levels of ozone above the Antarctic between 1980 and 1991:
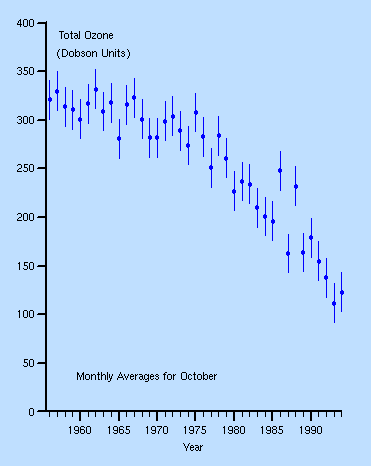
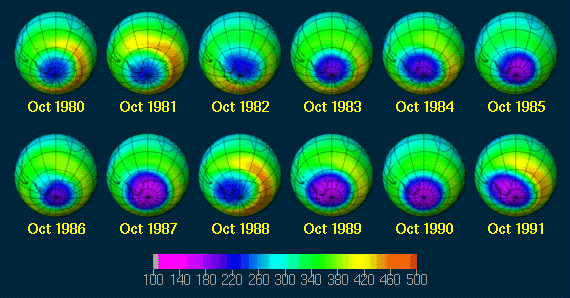
The reduction over time is clear. The pictures corresponding to the graph are below. They show how the ozone hole has grown over the past years. It is thought to continue growing until about 20 years after the complete phasing out of CFC's.
After much research by scientists all over the world governments decided that something had to be done to stop the ozone depletion of the ozone layer. In 1987 24 countries signed a treaty aiming to save the ozone layer. This Montreal Protocol called for a reduction in the production of ozone depleting substances, aiming to reduce CFC's by 50% by the year 2000. Since then alternatives have been found to fulfil the roles of CFC's and the amounts being released to the atmosphere have dramatically reduced. However the ozone hole is still a problem needing to be carefully monitored.
| THE CHEMICAL OZONE | LOW LEVEL OZONE |
| INFO. ON ME | LINKS |