![]()
Phenolphthalein
The acidity indicator and laxative
that resulted from two lucky accidents
![]()
![]()
Molecule of the Month March 2022
Also available: HTML version.
![]()

PhenolphthaleinThe acidity indicator and laxative
|
 |
The indicator part, or the laxative part?
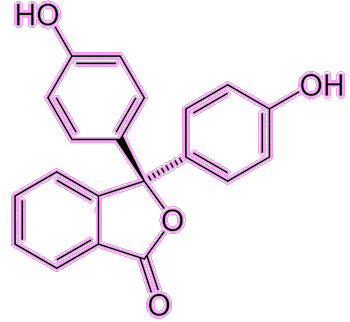
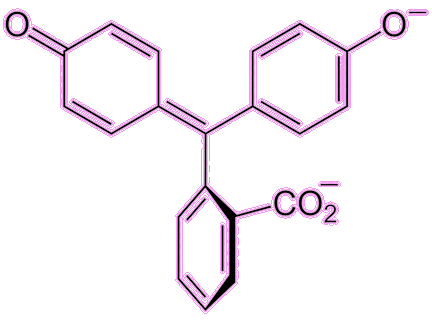
Well, the indicator part is well known from school chemistry experiments, as it is a molecule that changes colour with pH, indicating whether a liquid is acidic or alkaline (basic). Phenolphthalein (often abbreviated to phph) is colourless in acidic or near-neutral conditions (pH = 0-8.3) but turns a pinky-fuchsia colour in basic/alkaline conditions (pH = 8.3-10). There are other colour changes at more extreme conditions (red at pH < 0 and colourless again at pH > 10) but these are not as useful.
Under acidic conditions the phph molecule becomes protonated, while under basic conditions it becomes deprotonated. These changes affect the electronic structure of the molecule and hence the wavelength of light it absorbs. This colour change is rapid, and so phph is commonly used in titrations to show when an acidic solution has been neutralised by addition of base (or vice versa).
 |
 |
 |
| Phph under acidic conditions (colourless) | Beaker of acid left, and alkali (right) with phph indicator added. | Phph under basic conditions (colourless) |
That’s what Universal Indicator is for. This is a mixture of water, an alcohol and several different indicators that exhibits smooth colour changes over a wide range pH values. The mixture includes 1-propanol, sodium hydroxide, methyl red, bromothymol blue, sodium bisulfite, thymol blue and phenolphthalein.

The colours of universal indicator at different pH values, starting from acid on the left to alkaline on the right.
The violet and indigo colours for strongly alkaline solutions result from a combination of blue from thymol blue and fuchsia from phenolphthalein.
[Photo: MILOS ALEKSANDRIC, CC BY-SA 4.0 via Wikimedia Commons]
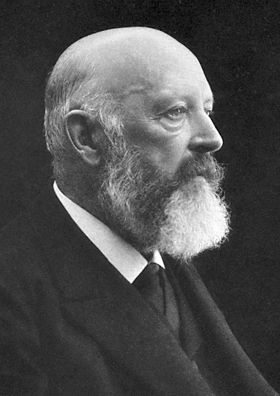 Not quite – but its use as an indicator was not its original intended use. In the late 1800s, William Henry Perkin made the first synthetic dye, mauvine dye (described in the first MOTM article in January 1996). This proved to be very popular in the clothing industry and extremely lucrative for the chemical industry, so the race was on to make more synthetic dyes. In 1871 in Germany, Adolf von Bayer (photo, right) was working on phthalein dyes, which were known to produce different colours. He reacted phthalic anhydride with phenol under acidic conditions, and produced a new molecule, phenolphthalein. This molecule wasn’t much use as a dye (too unstable), but luckily was discovered to be an excellent acid-base indicator. Baeyer received the Nobel Prize for Chemistry in 1905 for his work on synthetic dyes, including phph, indigotin (MOTM February 2009), and fluorescein.
Not quite – but its use as an indicator was not its original intended use. In the late 1800s, William Henry Perkin made the first synthetic dye, mauvine dye (described in the first MOTM article in January 1996). This proved to be very popular in the clothing industry and extremely lucrative for the chemical industry, so the race was on to make more synthetic dyes. In 1871 in Germany, Adolf von Bayer (photo, right) was working on phthalein dyes, which were known to produce different colours. He reacted phthalic anhydride with phenol under acidic conditions, and produced a new molecule, phenolphthalein. This molecule wasn’t much use as a dye (too unstable), but luckily was discovered to be an excellent acid-base indicator. Baeyer received the Nobel Prize for Chemistry in 1905 for his work on synthetic dyes, including phph, indigotin (MOTM February 2009), and fluorescein.
Yes, in 1900, shortly after the discovery of phph’s indicator properties, the authorities in Hungary were having a problem with their wines. The grape harvest had failed, and authentic Hungarian wines were in short supply and therefore expensive. Unscrupulous dealers were relabelling cheaper foreign wines and passing them off as Hungarian, much to the government’s annoyance. The government decided to thwart this activity by adding to the wine a substance which, while colourless itself, developed a characteristic colour upon the addition of some simple reagent. The newly discovered indicator, phph, being tasteless and colourless in acidic solutions (like most wines), seemed ideal. An authentic Hungarian white wine would then turn pink when a base was added, while a fake one would not.
The story goes that the government’s plan went ahead, but it was soon discovered that the addition of even tiny amount of phph to the wine was causing the drinkers to have acute diarrhoea within a couple of hours, and which lasted 1-2 days!
To test that the phph additions were safe for consumption, chemist Zoltan Vamossy from the University of Budapest had previously performed animal tests which indicated the compound was harmless, but later human trials (on Vamossy and his lab technicians!) showed it caused rapid onset of diarrhoea. Vamossy had “… discovered a laxative of great merit.” Other accounts say that the human tests were only performed after the wine had been sent out to the public, as a result of reports of a sudden outbreak of mass diarrhoea among the Hungarian wine-drinking population!
In any case, doctors soon heard about the new laxative, and began to prescribe it instead of the standard foul-tasting castor oil because it was easier to take, especially for children. The word got around, and commercial phph-containing laxatives (a.k.a. purgatives) called Purgen, Purgatin, Purgolade and Laxine became readily available in Germany and Eastern Europe.
 A paper in the British Medical Journal by F.W. Tunnicliffe from 1902 describes the effects of taking Purgen on various bodily excretions:
A paper in the British Medical Journal by F.W. Tunnicliffe from 1902 describes the effects of taking Purgen on various bodily excretions:
“Concerning the fate of phenolphthalein in the body, all that can be said is that it is only present as such in the urine, to a very slight degree, and only after very large doses have been taken. Its presence in the urine can, of course, easily be demonstrated. Upon adding a small quantity of alkali to such a urine it becomes at once either rose-red or deep purple according to the quantity of phenolphthalein present.
Whatever doubt there may be concerning the presence of minute quantities of Purgen or an immediative derivative in the urine of patients taking it, there can be no doubt whatever concerning its presence in the faeces. If a small quantity of alkali be added to the motions after Purgen has been given to a patient, the whole will quickly develop a brilliant purple colour. This reaction is present occasionally for one or two days after the exhibition of a single large dose!”
However, although phph solutions were much easier to take than caster oil, they were still not that palatable, and a tastier solution was needed.
 So, what happened?
So, what happened?In New York, a Hungarian-born pharmacist called Max Kiss had been following the stories of phph in Hungary. He realised that he could solve the bad-taste problem by blending phph with chocolate. The new product was called Ex-Lax, and the advertisements for it called it “A treat instead of torture“. The origin of the name is uncertain – some sources say it came from the phrase ‘excellent laxative’, while another says it came from a Hungarian word for ‘blockage’ or ‘deadlock’, which was being used in the country’s press at the time to describe their current parliamentary deadlock. Whatever the origin, when Ex-Lax was launched in 1906, it was rapidly acquired by the pharmaceutical company Novartis. For the next 90 years it was the best-selling laxative of all time – as well as being the source of many a student prank.
Throughout the 1950s and 60s a common prank by pupils would be to offer their teacher or other pupils a ‘chocolate’, in the hope that the rapid onset diarrhoea would lead to hilarious results – or maybe even a day off school. Needless to say,this is actually quite dangerous, and is not encouraged!
 Does Ex-Lax still exist?
Does Ex-Lax still exist?Yes, but the phph component has now been replaced by other molecules which have a laxative effect, such as sennosides extracted from the senna plant.
In the 1990s the US National Cancer Institute reported that rodents fed with huge amounts of phph developed cancer. Other animal experiments had also cast doubts on the safety of phph. But despite these animal experiments all being rather questionable, and not directly relevant to the tiny doses of phph found in laxative pills, the International Agency for Research on Cancer declared phph to be a Group 2B carcinogen, meaning that it ‘possibly causes cancer in humans’. It was banned as an over-the-counter laxative in the US and many European countries in 1997.
Yes, it’s used to test the durability of concrete.
Concrete is an alkaline material which has a pH of around 12 to 13. The steel reinforcing bars within the concrete are stable in this alkaline environment and do not corrode. But when carbon dioxide from the air dissolves in water trapped in pores in the concrete, it creates carbonic acid, which then reacts with the concrete, reducing its alkalinity. When the pH of concrete surrounding the steel bars decreases to below 9.5, they begin to corrode, and in doing so expand a little bit. The volume increase causes the concrete to crack and eventually crumble. This is called concrete carbonation, sometimes called ‘concrete cancer’ in the popular press.
To find the depth of carbonation, an engineer cuts a piece of the concrete then sprays it with phph solution. The healthy alkaline parts show up as pink, whereas the damaged acidic regions will show no colour change.
 |
 |
| Carbonation causing crumbing of the concrete in the walls of a car park. [Image: Carboncure.com] |
Analysing carbonation using phph. [Image from: Civilengineeringforum] |
It’s also used in magic tricks.
 How?
How?The most famous is the ‘turn water into wine’ trick, which you can see demonstrated in this YouTube video. Take an empty wine glass and a jug of water. Pour the water from the jug into the first empty glass, and it will stay colourless. But pour water from the same jug into the second empty glass and it will instantly turn red, like wine. Magic!? No, just chemistry!
Ah, it does require some preparation. First, the jug of water doesn’t just contain water – it’s a slightly alkaline solution made up beforehand by mixing one teaspoon of sodium carbonate powder into a pint of water, and stirring until clear. Next, the second ‘empty’ glass isn’t entirely empty – it contains a teaspoon of phph solution. When the ‘water’ is poured into the first empty glass, it stays clear. But when the ‘water’ (really alkaline carbonate solution) is poured into the second seemingly empty glass (containing phph), it turns pink. But don’t let anyone drink the ‘red wine’, or the magic trick may end with a rapid sprint to the nearest toilet!
I think I also saw phenolphthalein mentioned in an episode of CSI or Dexter…Possibly – phph is a component of the Kastle–Meyer test used to detect the presence of blood. In 1901, the American chemist Joseph Hoeing Kastle discovered that haemoglobin in blood could oxidise mildly alkaline colourless phph solutions, turning them pink. Two years later, the German chemist, Erich Meyer modified the crude test and turned it into a forensic tool. Although it cannot distinguish between human and animal blood, the Kastle-Meyer test is used by police forces all over the world as a quick test for the presence of blood at a crime scene. |
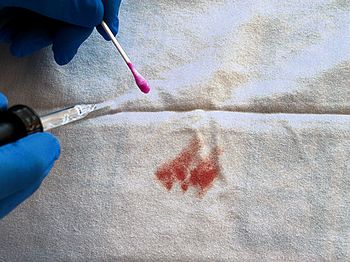 The Kastle-Meyer test gives a pink colour in the presence of blood. |
 |
Can this colour change be used for anything else?Yes, it was used in WW2 as an ‘invisible ink’ for sending secret messages. How did that work?The message is written using phph ‘ink’ dissolved in slightly alcoholic water (pH ~ 7) using a fountain pen. This is invisible on the page because the phph is colourless at this pH. But if the paper is sprayed with a slightly alkaline solution (e.g. ammonia or sodium bicarbonate), the pink letters reveal themselves. Spraying it with a slightly acid solution (e.g. vinegar) makes the message invisible again. See this YouTube video for a demonstration of this. |
 Any other examples?
Any other examples?One version of the Barbie doll had ‘Hollywood Hair’, which turned pink when sprayed with a ‘magic’ liquid. This works because the pigment which coloured Barbie’s hair blonde had been mixed with a little phph. When the magic spray (which was a dilute alkaline NaOH solution) was sprayed onto the hair, the indicator turned it pink. The liquid could be sprayed through a stencil to make pink patterns in the hair. A similar indicator molecule to phph, thymolphthalein, was used for a blue colour.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16811158]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16811158]