![]()
Phosphine
The fishy smelling insecticide
![]()
Glenny Wu and Stephen Belding
Rugby School, UK
![]()
Molecule of the Month November 2024
Also available: JSMol version.
![]()
PhosphineThe fishy smelling insecticide
Glenny Wu and Stephen Belding
Molecule of the Month November 2024
|
 |
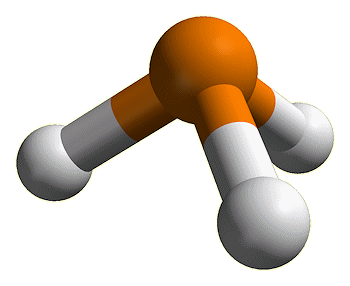
Phosphine, also known as hydrogen phosphide, is a colourless, flammable, and highly toxic gas with the chemical formula PH3. It has a distinct, unpleasant odour often compared to garlic or rotting fish. Despite its pungent smell and toxicity, phosphine is an essential compound in various industrial and chemical applications.
 Sir Humphry Davy [Image: James Lonsdale, Public domain, via Wikimedia Commons] |
How was phosphine discovered?Phosphine was first identified by the English chemist Sir Humphry Davy in 1812. Davy, exploring the chemistry of phosphorus and its compounds, reacted calcium phosphide (Ca3P2) with water, and observed the production of a flammable gas, later identified as phosphine. The reaction is as follows: Ca3P2(s) + 6H2O(l) Davy's discovery laid the foundation for understanding phosphine's chemical behaviour and applications. Initially, phosphine was of scientific curiosity due to its pyrophoric (spontaneously igniting) nature. This property is actually due to traces of diphosphine (P2H4) (see later). The phenomenon of phosphine smoke rings, often referred to as vortices, provided a dramatic demonstration of its reactivity. |
The smoke ring demonstration clearly shows the reactivity of phosphine. To make your own demonstration in a lab you will need:
1. Chemicals:
2. Apparatus:
3. Method:
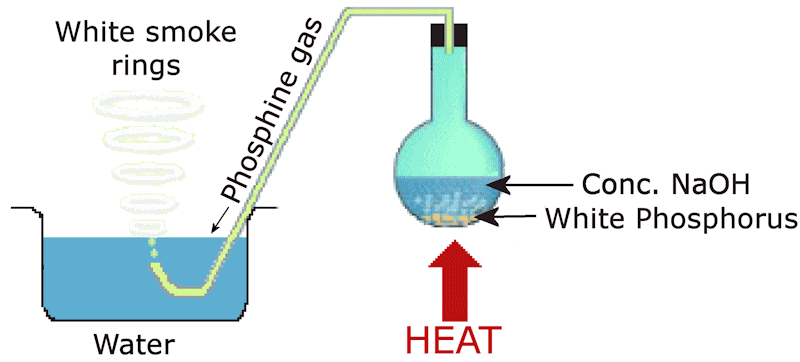
The phosphine smoke-ring demonstration
This demonstration is an interesting example of a disproportionation reaction. The oxidation states of phosphorus are: P4 (0); PH3 (-3) and NaH2PO2 (+1).
3 NaOH + P + 3 H2O  PH3 + 3 NaH2PO2
PH3 + 3 NaH2PO2
4. The burning phosphine generates phosphorus pentoxide (P4O10):
4 PH3 + 8 O2 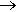 6 H2O + P4O10
6 H2O + P4O10
The white smoke rings are caused by the very high solubility of phosphorus pentoxide, leading the water vapour in the air to condense into a fine mist.
Isn’t this reaction in…?Yes, this reaction features in Breaking Bad. In the first episode, Walter White uses red phosphorus in boiling water to create phosphine gas and poison two rival gangsters (as shown in this YouTube video). However, in reality, this reaction would actually need white phosphorus, and the water would also have to contain sodium hydroxide! |
 Walter White makes phosphine in Breaking Bad. |
Due to the phase-out of methyl bromide in some countries under the Montreal Protocol, phosphine is now the main cost-effective fumigant that works quickly without leaving residues on stored goods, particularly grain stores. It is sold in the form of aluminium phosphide tablets (AlP), known as 'wheat pills'. Phosphine is released when these are added to water:
2 AlP + 3 H2O  Al2O3 + 2 PH3
Al2O3 + 2 PH3
The phosphine has a very low concentration; high enough to kill insect pests, but low enough to avoid an explosion. Ammonium carbamate is also added to the pills. In the heat from the above reaction, this decomposes to produce ammonia and carbon dioxide, which also help mitigate the explosion risk.
[NH4][NH2CO2] 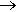 2 NH3 + CO2
2 NH3 + CO2
The use of this pesticide can be rather dangerous in inexperienced hands. In the UK in 2021, there was a tragic case in which the reaction was mistakenly used to fumigate a residential property. A woman had illegally imported aluminium phosphide from Italy to deal with a bedbug infestation in her flat in Shadwell, East London. But she had not read the packaging, and distributed a deadly amount around her flat, and then left the flat for 24 hours for the fumigation to work. But the amount of phosphine produced was too great, and some of it seeped into a neighbouring flat, killing an 11-year-old girl and causing another young child to be hospitalised.
Is aluminium phosphide banned?Several countries have banned the use of AlP as an insecticide or rodenticide, but many still allow it because it is a very effective way of fumigating wheat and grain, and so long as it's used by trained personnel it's generally safe. However, this hasn't stopped it becoming a major source of poisonings, both accidental and deliberate. India, in particular, suffered a huge increase in AlP poisonings, some of which have rather odd side-effects... Such as...?If ingested, the acid in the stomach causes the release of phosphine gas, which under some rare circumstances, can self-ignite! There are even reports of blue flames erupting from the end of syringes being used by doctors trying to suck out AlP tablets from a patient's stomach in hospital Emergency rooms! |
 Aluminium phosphide tablets [Image:همان, CC BY-SA 3.0 via Wikimedia Commons] |
It's used in the semiconductor industry to incorporate P atoms as an impurity into silicon to make it into an n-type semiconducting material. Together with p-type material (made by adding boron to Si), they can make p-n junctions, which are the building blocks of transistor circuits and microprocessors.
Phosphine and its derivatives are also used to fabricate gallium phosphide (GaP), which is another semiconducting material used to make low-cost red, orange, and green light-emitting diodes (LEDs) since the 1960s.
Various LEDs are made from GaP. Note that blue LEDs are much newer and are made from gallium nitride (see the MOTM for February 2023).
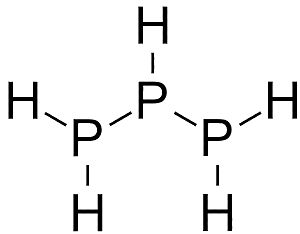
Yes. Phosphanes are saturated phosphorus hydrides with general formula PnHn+2, one example being triphosphane. Diphosphine (P2H4) is an impurity in the synthesis of phosphine and is responsible for its pyrophoric properties. This byproduct of phosphine synthesis is much more common when Ca3P2 is used as a reactant: this substance already contains a P-P bond. It is likely that the reactivity of diphosphine is due to the very weak P-P bond. For comparison, the P-H bond strength is 322 kJ mol-1, while P-P is only 201 kJ mol-1. And there are many organophosphines, which are made from PH3 by substituting one or more hydrogen atoms with organic groups, such as methyl, to make methylphosphine.
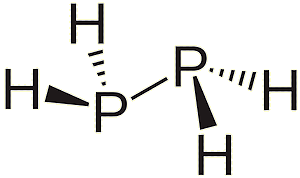 |
 |
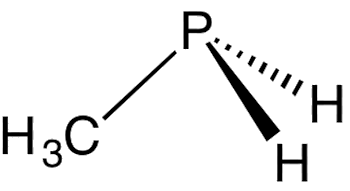 |
| Diphosphine | Triphosphane | Methylphosphine |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27132018]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27132018]