


A version of this article originally appeared in The Alchemist on ChemWeb.com in October 2002.

Pnicogen is perhaps one of the most mispronounced and misspelled chemical terms since phthalocyanine or possible phenolphthalein. "As esoteric as it may seem, a lively discussion about the pronunciation and use of "pnicogen" and "pnictide" did transpire at a recent Gordon conference," solid state chemist Arthur Mar of the University of Alberta, Edmonton, told me. So, I did a quick web search and found about 100 pages that mention 'pnicogen' while some 250 say 'pnictogen'.
Even a simple search on ScienceDirect shows that there are about 25 pnictogen papers and ten on pnicogens, so it's about 2.5 to 1 for pnictogen. The pnicogen group of elements, named from the Greek word for "choking"*, however, could, regardless of spelling and etymology, provide technologists with a whole new range of materials to play with. Applications might range from novel semiconductor devices to non-linear optical materials offering light splitting and frequency doubling effects**, solid-state refrigeration and air conditioning and higher density computer hard disks.
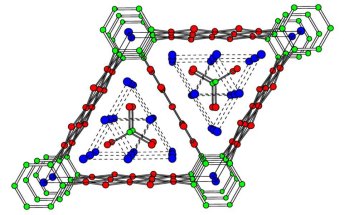
The structure of La13Ga8Sb21 - taken from the Oct 2002 Catalyst pages
(original image courtesy of Arthur Mar of the University of Alberta).
The pnictides - compounds of phosphorus, arsenic, antimony, and bismuth - form all kinds of exotic materials. Some are diamagnetic paramagnets at room temperature, others are antiferromagnetic or ferromagnetic but at low temperatures. Some are transparent, others refractory. Some have holes where you'd expect an electron and yet others produce electricity when they get hot. The pnictides form a diverse range of semimetals and semiconductors.
One particular sub-group comes into focus in a recent issue of Coordination Chemistry Reviews - the ternary rare-earth main-group pnictides. These materials have the following structure - RExMyPnz. The M represents a Group 13 or 14 element. Pn is a pnictide element (P, As, Sb, Bi; N is not included because it throws up a whole different array of chemicals and properties). These materials occupy the limbo between ionic and covalent materials and should you encounter them you can expect to consign any and all the usual rules of bonding to the recycling bin because as their unfamiliar seeming formulae suggest, they are as bizarre in behaviour as they are in name.
Bizarre is as bizarre does though, and this emergent area of solid-state chemistry pioneered by Wolfgang Jeitschko emeritus of the University Münster, Germany, and others, is demonstrating just how new vistas in the inorganic world can appear. Organic chemists might feel they have the edge on variety with their multitude of materials (the CAS registry is (at the time of writing) up to 48,114,528 chemical substance registrations, it was a "mere" 30 million or so when I mentioned it last in my Catalyst column of May, 2001! However, never mind the quantity feel the width? - of the bonds that is. Whereas as rule of thumb organic compounds follow a reasonably simple bonding scheme, as Mar and colleagues point out in the Coordination Chemistry Review, for most combinations of the elements the electronegativity differences between partners in a compound are intermediate and "the resulting structures display a bewildering variety of bonding patterns."
Indeed, the pnictides offer some of the more interesting examples allowing themselves to form extended solid-state compounds that fall between the two extremes of ionic and intermetallic materials. Sodium chloride one might consider as the archetype ionic with its electrons akimbo and an intermetallic material like Cu5Zn8 where the partners are almost equal and the electrons exist in the shared valence band. The result is a group of materials often with homoatomic M-M bonds and bonds directly between the pnictide components. And it is, of course, their unusual bonding that endows these materials with their extraordinary electromagnetic properties.
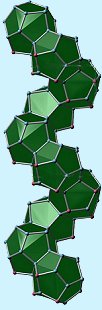 The structure of K6Bi2Sn23 (from the University of Darmstadt) |
Mar and his colleagues began investigating ternary compounds with the format RE-M-Pn, where M is a Group 13 or 14 element and Pn is a pnicogen (P, As, Sb, Bi). Their main focus has been on the antimonides with potential as superconductors and several have already been identified. As they emphasise, earlier work by Brigitte Eisenmann and Gerhard Cordier of the Technical University of Darmstadt, Germany, and A. Schäfer had demonstrated that there was a vast diversity of chemistry to investigate among the ternary alkali and alkaline-earth main-group pnictides, with fractional bond orders being the order of the day. So, a compound, as typical as is possible, investigated by the team might be LaSiP3, Eu2Ga2P6 or K6Bi2Sn23 (shown left). What may come as a surprise to some, and simply be put down to Sod's Law by others, is that several of these materials, such as the rare-earth silicon phosphides and arsenides were originally discovered as the mere byproducts of high temperature chemistry reactions in silica tubes. But, serendipity aside, the design and investigation of pnictides is moving apace. |
By now, you may have come to expect surprises from the pnictides. Indeed, Susan Kauzlarich and her colleagues at the University of California Davis are investigating complex layered pnictide-oxide compounds. "This is a large and diverse field of research, both solid state and solution chemists work with these elements to produce novel materials with unique bonding, Kauzlarich told us, "Several theory groups are also interested in these materials."
Kauzlarich and her team work with ternary pnictides and pnictide oxides. They have recently synthesized and characterized new compounds with a Ba2Mn3As2O2. These materials, transition metal pnictide oxides, she points out are unlike other pnicogen-containing compounds in that there are no pnicogen-oxygen bonding interactions. Instead, they have observed layered materials, which Kauzarlich says might offer unique properties because of their unique crystalline structure type, perhaps providing opportunities in semiconductors, physics of low-dimensional electronic and magnetic materials and high temperature superconductivity.
The team has also recently published the X-ray magnetic circular dichroism (XMCD) spectrum of another pnictide, Yb14MnSb11, and revealed a small dichroism effect indicative of an anti-alignment of a small moment on Sb with the moment on Mn. This, the researchers suggest, supports the prediction of a hole on the pnictide, another anomalous bonding property that might be exploited in novel magnetic materials. Indeed, the group has, says Mar, "been instrumental in identifying colossal magnetoresistance properties in the Eu14MnSb11-related family of compounds - with potential for applications in magnetic read-write heads.
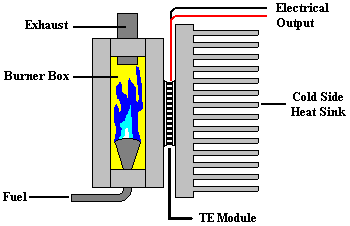 |
A typical thermoelectric generator (TEG) used to convert temperature differences into electrical power. There are four basic components: a heat source (which in the case shown is a burner box with a propane fuel source), a TEG module (i.e., a thermoelectric generator — also known as a Seebeck device), a ‘cold-side’ heat sink, and the electrical load. The system may also include a voltage regulation circuit, or a fan for the heat sink. Image from theTellurex website. |
Holger Kleinke of the University of Waterloo in Ontario, meanwhile, is developing pnictides with thermoelectric properties. Such materials are capable of converting heat into electrical energy and vice versa, by definition. These materials may find use in power generators (see diagram above) in telecommunications devices and on spacecraft, for instance . They might also be useful in food refrigeration and air conditioning (see diagram below) because they could offer an environmentally friendly way to heat or cool that does not involve the pumped evaporation and condensation of a volatile fluid. Thermoelectric materials can also be used in cryotherapy, pacemakers, and as thermocouple sensors. One problem is the low conversion efficiency currently seen. Kleinke suggests that once the efficiencies improve, environmentally harmless air conditioning or power supplies for an in-car stereo or headlights could be driven by heat from the vehicle's exhaust.
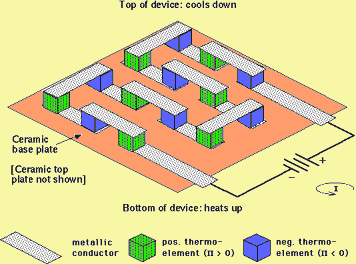 |
A thermoelectic cooling module, which is the opposite of a TEG - it takes electrical power and produces a temperature difference, i.e. cooling. An array of positive and negative thermoelements (possibly made from a pnictide) are arranged between two ceramic plates so that they are electrically in series but thermally in parallel. All of the elements move heat from the top to the bottom of the module. Note that all of the metallic conductors are entirely in the plane of the top plate or the bottom plate. |
By starting with the poor thermoelectric binary antimonide Mo3SbM7, and then adding two more valence electrons to the structure by partial substitution of the antimony with tellurium, the Waterloo team were able to create a new material, "without noticeable structural changes". This substitution, they say, has led to "a dramatic enhancement in the thermopower, indicating that semiconducting properties have been achieved." This promising thermoelectric pnictide material Mo3Sb5Te2, with further development, hopefully will provide a foundation for high efficiency materials that might one day compete with freon refrigeration and other heat conventional exchange technologies.
Mercouri Kanatzidis' group at Michigan State University is also working on related materials together with Tellurex. Their efforts are aimed at synthesizing bulk materials with higher figures of merit than those attainable with the thermoelectric Bi2Te3. These materials could eventually find use in solid-state refrigerators, too.
Perhaps the seeming ambiguity and confusion that surrounds the term pnicogen and the fact that IUPAC does not recommend its use at all, somehow hint at the strangeness of the pnictide compounds, but whether you say pnictogen and I say pnicogen, it will be a long time before anyone calls the whole thing off.
References:
Footnotes
* The term pnicogen is not actually recommended by IUPAC, nevertheless, use of the Greek verb "to choke" may have one of many origins. Perhaps it refers to the fact that nitrogen is a "choking gas" or that PH3 gives you a gagging reflex. "pnigo; pnigein" by definition is to choke, throttle, wring the neck of, strangle. If any readers know any more of the etymology of pnicogens, I would be interested to hear from them; email me via my website (www.sciencebase.com).
** Walter Lambrecht and colleagues at Case Western Reserve University, Ohio, are working on pnictides and chalcogenides with non-linear optical properties that could be used as frequency doublers for laser light.

![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437270]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437270]