 |
PROPANETHIAL S-OXIDEThe lachrymatory factor in onions |
 |
 |
PROPANETHIAL S-OXIDEThe lachrymatory factor in onions |
 |
Paul M. Burnham
Hillsborough College, Sheffield, UK
Also available: HTML-only, VRML and JMol versions.
 |
The Egyptians valued onions highly and they were used as a form of currency to pay workers who built the pyramids. They were also a symbol of eternity, because of their concentric layer structure, and a basket of onions was considered a very respectable funeral offering. Many of the pharaohs were buried with onions and archaeologists discovered small onions in the eye sockets of King Ramesses IV’s mummy. |
| EYE WATERING CHEMISTRY | ||||
It is well known that people ‘cry’ when chopping onions but why is this so? The answer is that propanethial S-oxide (often referred to as thiopropanal S-oxide) is released into the air during chopping. Propanethial S-oxide is a lachrymator, an irritant that causes the eyes to fill with tears without damaging them. When a lachrymator comes into contact with the surface of the eye, the cornea, it is detected by the nervous system and triggers a response from the lachrymal (tear) glands. Tears are then produced in order to dilute the irritant. Propanethial S-oxide is relatively volatile and when its vapours come into contact with the eye a small amount reacts to form sulfuric acid, causing the burning and itching sensations that accompany the tears. |
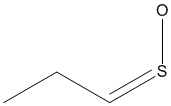 |
| Propanethial S-oxide, C3H6SO |
Interestingly, onions do not contain propanethial S-oxide and it is thought that onions produce this tear inducing compound to protect the plant from herbivores. It is the product of a series of chemical reactions, shown below, that occur once the onion has been damaged. Onions have many sulfur containing molecules within them, but the precursor to propanethial S-oxide has been identified as the amino acid S-1-propenyl-L-cysteine sulfoxide (which is very similar to the chemical alliin, found in garlic). Cells are broken open as the onion is cut and this releases the enzyme alliinase and water, which react with S-1-propenyl-L-cysteine sulfoxide forming a mixture of products. These products are the precursors for a variety of compounds that form the flavour of onions and include 1-propenyl sulfenic acid. The lachrymator propanethial S-oxide is formed from 1-propenyl sulfenic acid in an enzyme catalysed reaction. It was originally thought that alliinase was responsible for this reaction. However, five years ago scientists in Japan identified another enzyme present in onions that carried out this process and named it lachrymatory-factor synthase. |
|
| Reaction scheme for the production of propanethial. |
| NO MORE TEARS? | |
Now that scientists understand the pathway for the production of propanethial S-oxide, research is being focussed on the production of genetically modified onions that do not contain the lachrymatory-factor synthase enzyme and hence will lead to tear-free cutting. However, many people have devised their own techniques to prevent ‘crying’. These include trying to breath in the lachrymator to prevent it from reaching the eyes. For example, cutting an onion while holding an object, such as a teaspoon or a piece of bread, in the mouth. |
 |
Another suggestion is to cool the onion prior to chopping. The theory behind this method is that less of the volatile propanethial S-oxide will evaporate, reducing the amount that reaches the eye. Alternatively, heating the onion prior to chopping may also reduce tearing by denaturing the enzymes present, preventing the formation of the irritant. A method for those who fancy a challenge is to hold the onion under water whilst chopping it. Allowing any propanethial S-oxide formed to react before it reaches the eyes. Opinion on the efficacy of these methods is varied and they have been discounted by many people. So much so that one American company, Broadway Panhandler, has developed onion goggles (pictured above). These glasses have a foam seal to prevent any vapours from entering the eye. Perhaps the most sensible suggestion is to use a very sharp knife so as to minimise cell damage. A step-by-step guide to chopping is available here. |
| BIBLIOGRAPHY |
|