![]()
Pyridine
The Pungent Powerhouse of Chemistry
![]()
Shanqiao Yang and Stephen Belding
Rugby School, UK
![]()
Molecule of the Month July 2025
Also available: JSMol version.
![]()

[Images: Modified from a design by Freepik]
PyridineThe Pungent Powerhouse of Chemistry
Shanqiao Yang and Stephen Belding
Molecule of the Month July 2025
|
 [Images: Modified from a design by Freepik] |
Pungent - in what way?It is infamous for its strong, unpleasant fishy odour. What's it like?It is a colourless liquid at room temperature, with a relatively low melting point of 232 K and high boiling point of 388 K. But it is also highly flammable, which aligns with the origin of its name. The term "pyridine" comes from the Greek word "pyro" meaning fire, a nod to its flammability. It has the chemical formula C5H5N, and is an aromatic compound. Structurally, it resembles benzene but with one key difference: in pyridine, one CH group is replaced by a nitrogen atom. This nitrogen atom imparts distinctive properties to pyridine, making it a crucial compound in both organic chemistry and industrial applications. This molecule is often described as being ‘heterocyclic’ due to the presence of the nitrogen atom. So it's aromatic in the same way as benzene?Yes. Pyridine possesses a conjugated system of six pi-electrons that are delocalised over its ring structure. This delocalisation occurs across the entire planar molecule, which ensures its stability and classifies it as aromatic. Pyridine follows Hückel's rule for aromaticity, which states that a molecule must have 4n+2 pi-electrons (where n is an integer) to be aromatic. In the case of pyridine, the six pi-electrons fit this rule, confirming its aromatic nature. |
 Bottle of pyridine [Image: LHcheM, CC BY-SA 3.0, via Wikimedia Commons] |
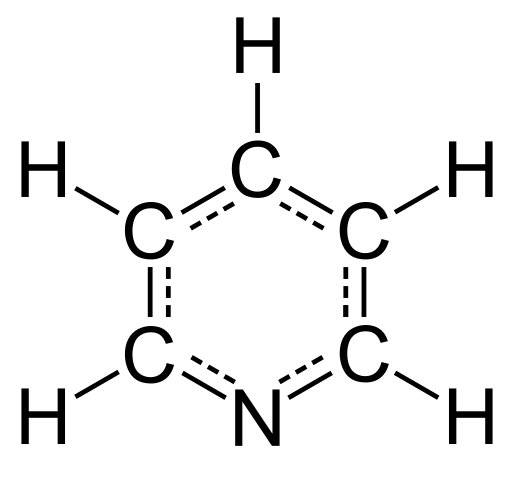
Delocalised structure of Pyridine
[Image: Jynto via Wikimedia Commons]
Well, one use is to help you avoid paying tax?
Pyridine is sometimes used as an alcohol denaturant! By adding pyridine, which is toxic and has a very unpleasant odour, to ethanol, the alcohol becomes undrinkable. This process of denaturing alcohol allows it to be safely used for industrial, laboratory, or other non-consumable purposes without being subject to the higher taxes and controls that are placed on alcoholic beverages. You may be more familiar with a similar process involving adding methanol together with a blue dye to ethanol, and the mixture is sold in shops as 'methylated spirits' for cleaning paintbrushes, etc.
In 1849, Scottish chemist Thomas Anderson successfully isolated pyridine from coal-tar oil. Coal-tar contains a very low concentration of pyridine, roughly 0.1%, so it required a multi-step purification process to isolate it effectively. Later, in 1869, the molecular structure of pyridine was determined by chemists Wilhelm Körner and James Dewar.
This discovery came just three years after Friedrich August Kekulé proposed the ring structure of benzene in 1866, marking a significant advancement in understanding aromatic compounds. However, determining the structure of pyridine wasn't so straightforward, and caused a few arguments at the time.
 |
 |
| Thomas Anderson (1819-1874) [[Image: Unknown photographer, Public domain, via Wikimedia Commons]] |
Stamp of Kekulé, in honour of the 150th anniversary of his birth. [Image: Nadia Kittel, Public domain, via Wikimedia Commons] |
Wilhelm Körner and James Dewar were two friends and chemists who both worked on determining pyridine's structure. However, their friendship faced a dispute over who first proposed the structure correctly. Körner is generally credited with being the first, having published his findings in 1869 in an Italian journal, Giornale di scienze naturali ed economiche. Dewar, on the other hand, mentioned the structure in a paper presented at the Royal Society of Edinburgh in 1870. Despite this being a year later, Dewar accused Körner of stealing his idea, leading to a controversy between the two.
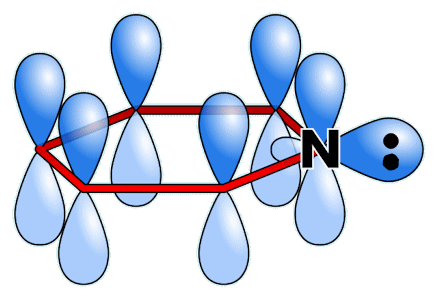 Lone pair on pyridine. [Image: Sponk (talk), Public domain, via Wikimedia Commons] |
Pyridine and benzene share structural similarities, as pyridine is isoelectronic with benzene, meaning they have the same number of electrons. However, their chemical properties differ significantly. Benzene, like other hydrocarbons, is neutral in terms of acid-base behaviour. In contrast, due to the presence of a nitrogen atom, pyridine acts as a weak base. Pyridine is less aromatic than benzene because the electronegative nitrogen atom disrupts the delocalisation in the ring. Nitrogen participates in the pi-bonding aromatic system using its unhybridised p-orbital, but its lone pair of electrons is in an sp2 orbital, projecting outward from the ring in the same plane as the σ-bonds (see diagram). This lone pair, therefore, does not contribute to the aromatic system. In terms of aromatic stabilisation energy, benzene is more stabilised due to its more effective delocalisation of pi-electrons, with a stabilisation energy of about 152 kJ/mol. Pyridine, on the other hand, is less stabilised, with an aromatic stabilisation energy around 117 kJ/mol. This difference is attributed to the electronegative nitrogen atom disrupting the electron delocalisation, reducing the overall aromaticity in pyridine compared to benzene. |
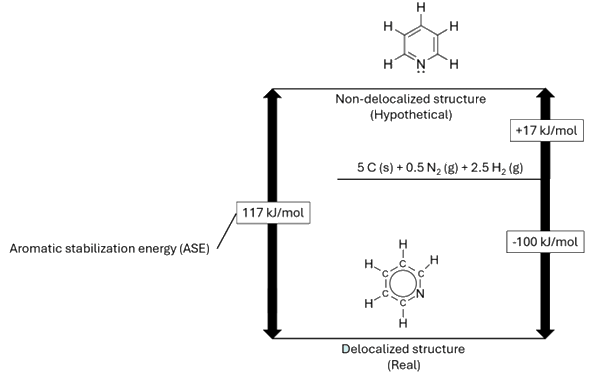
Energy level diagram showing the standard enthalpy of formations of pyridine and the
hypothetical pyridine structure without any delocalised electrons.
The delocalised form is more stable by 117 kJ/mol.
[Image: Self-drawn. Values cited from Hubbard, W.N.; Frow, F.R.; Waddington, G., J. Phys. Chem., 1961, 65, 1326
and Joule, J. A.; Mills, K. (2010). Heterocyclic Chemistry (5th ed.), Blackwell Publishing, Chichester UK.]
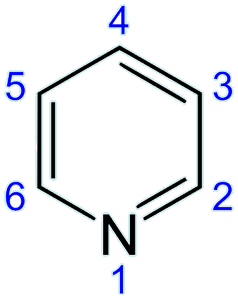 Numbering on pyridine. [Image: Yikrazuul, Public domain, via Wikimedia Commons] |
So they must react differently?Yes. Both pyridine and benzene undergo electrophilic substitution reactions, but pyridine reacts much more slowly, with a relative rate of 1×10-12! This is mainly because the electronegative N atom in pyridine withdraws electrons away from the aromatic ring, making it less available to electrophilic attack. As a result, very extreme conditions are required for this reaction to occur. Substitution normally takes place on carbon 3 and carbon 5 as this avoids a positive charge on the N atom of the carbocation intermediate. But unlike benzene, pyridine can undergo nucleophilic addition-elimination reactions. One example of this is the Chichibabin reaction, and the mechanism is shown below. |
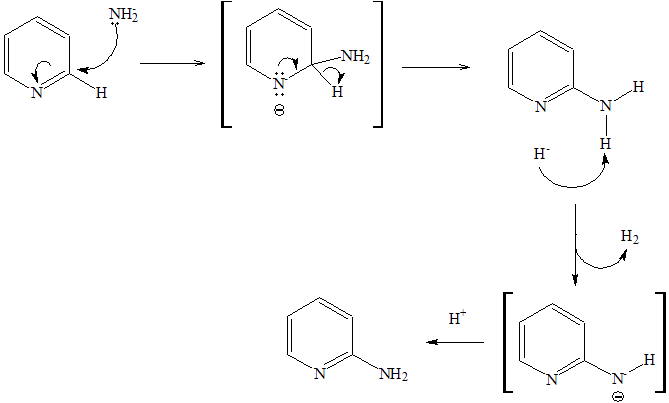
Mechanism of Chichibabin Reaction.
[Image: Paginazero, Public domain, via Wikimedia Commons]
The pKa value is a measure of a compound's basic strength; the higher the pKa value, the stronger the base. This means that a compound with a high pKa has a lone pair of electrons on its nitrogen atom that is more readily available to form a dative bond with a proton (H+). Here’s a league table that shows how pyridine compares with other amines:
|
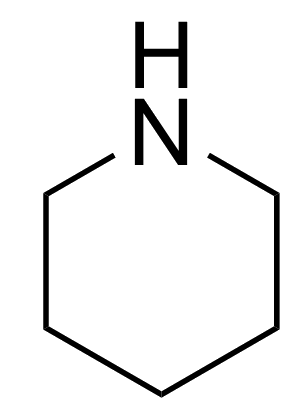 Piperidine |
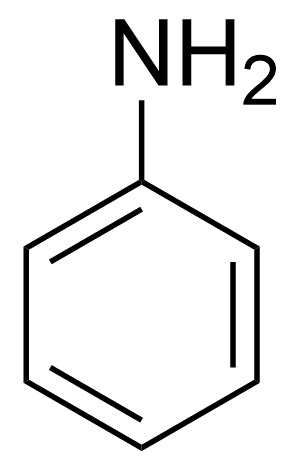 Aniline |
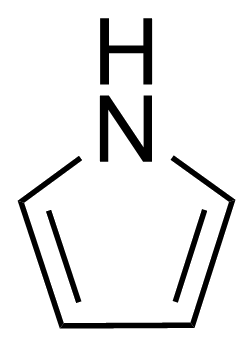 Pyrrole |
The molecules above pyridine in the table , for example piperidine, contain sp3-hybridised nitrogen atoms, which affects their basicity. Pyridine's moderate pKa is due to the nitrogen being part of the aromatic ring, which makes its lone-pair of electrons less available for protonation. With sp2-hybridisation, the nitrogen atom in pyridine has a higher s-character (33%) compared to the lower s-character (25%) of sp3-hybridised nitrogens in the molecules above pyridine. This results in pyridine's lone-pair being more strongly attracted to the N nucleus, making it less available and less likely to accept a proton.
In contrast, the molecules below pyridine, aniline and pyrrole, feature lone-pairs on nitrogen that are actually part of the delocalised pi-electron system of their aromatic structures. Protonating these lone pairs disrupts aromaticity, which is energetically unfavourable.
Since pyridine is an effective solvent, it is often commercially used in paint, rubber and dye production as a solvent. However, pyridine is more regularly used as a precursor for herbicides, for example, paraquat. This is one of the world’s most commonly used herbicides. Reaction of synthesis of paraquat from pyridine is shown as below.
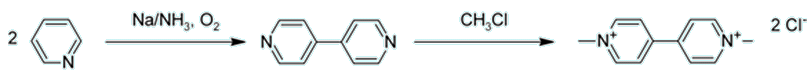
Synthesis of paraquat.
[Image: Rifleman 82, Public domain via Wikimedia Commons]
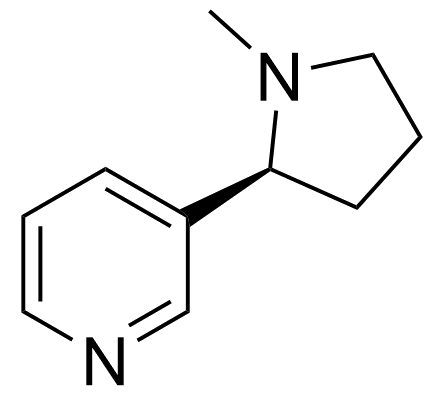
Pyridine is also very similar to the molecule nicotine (MOTM for August 2001), the active and addictive ingredient found in cigarettes.
 |
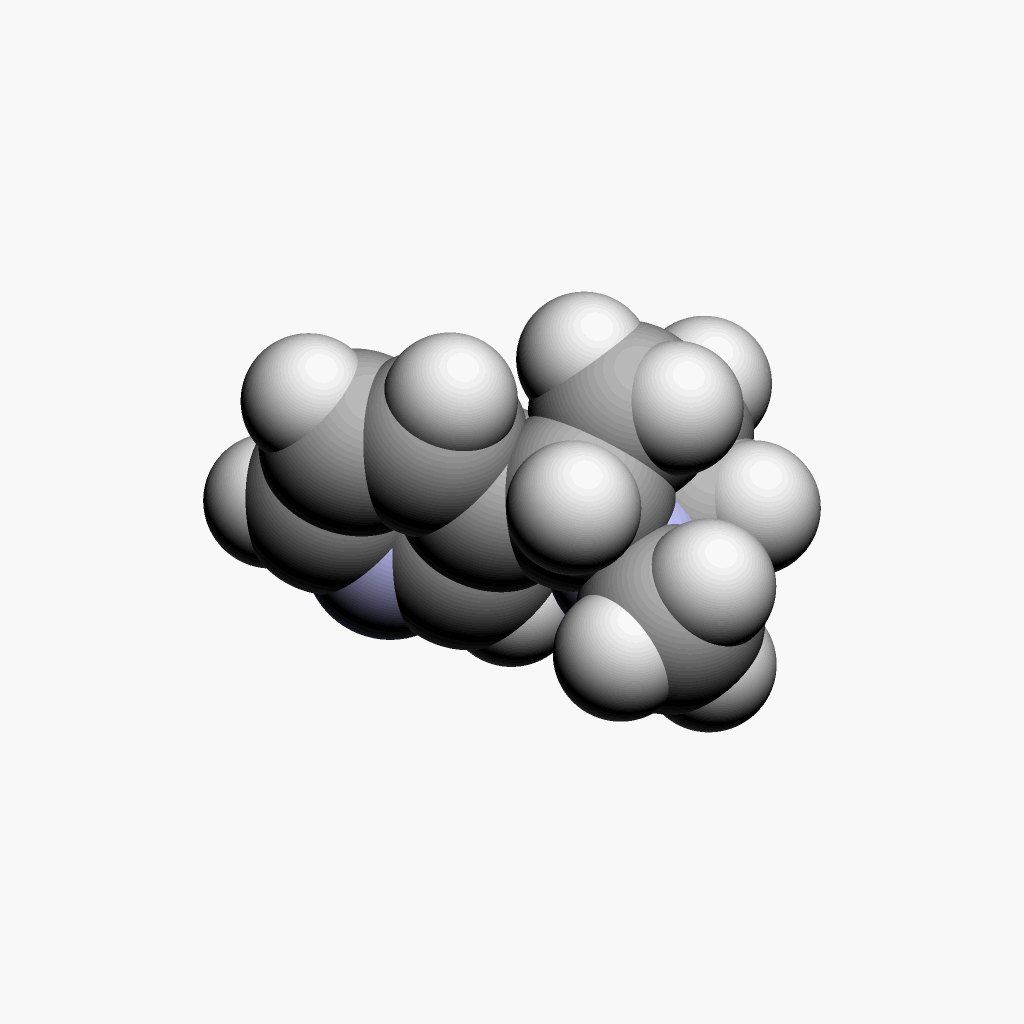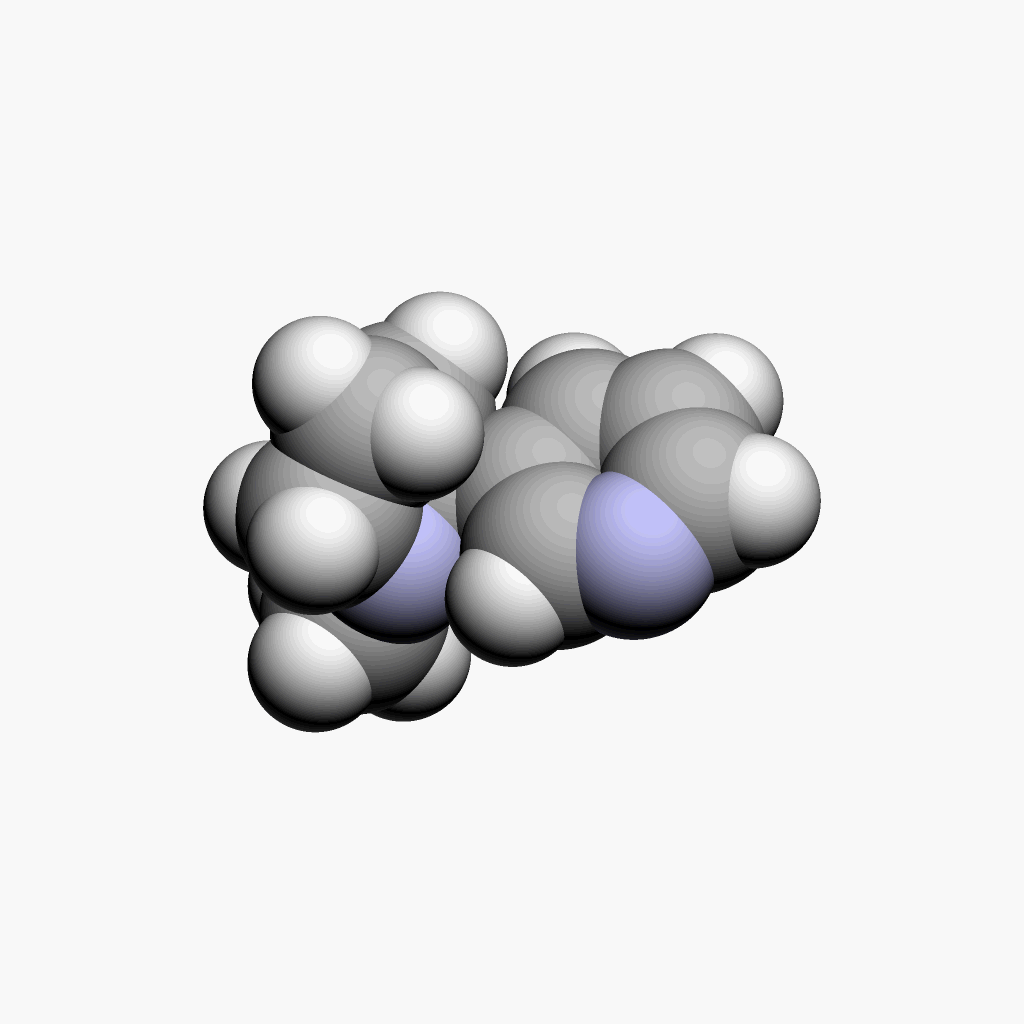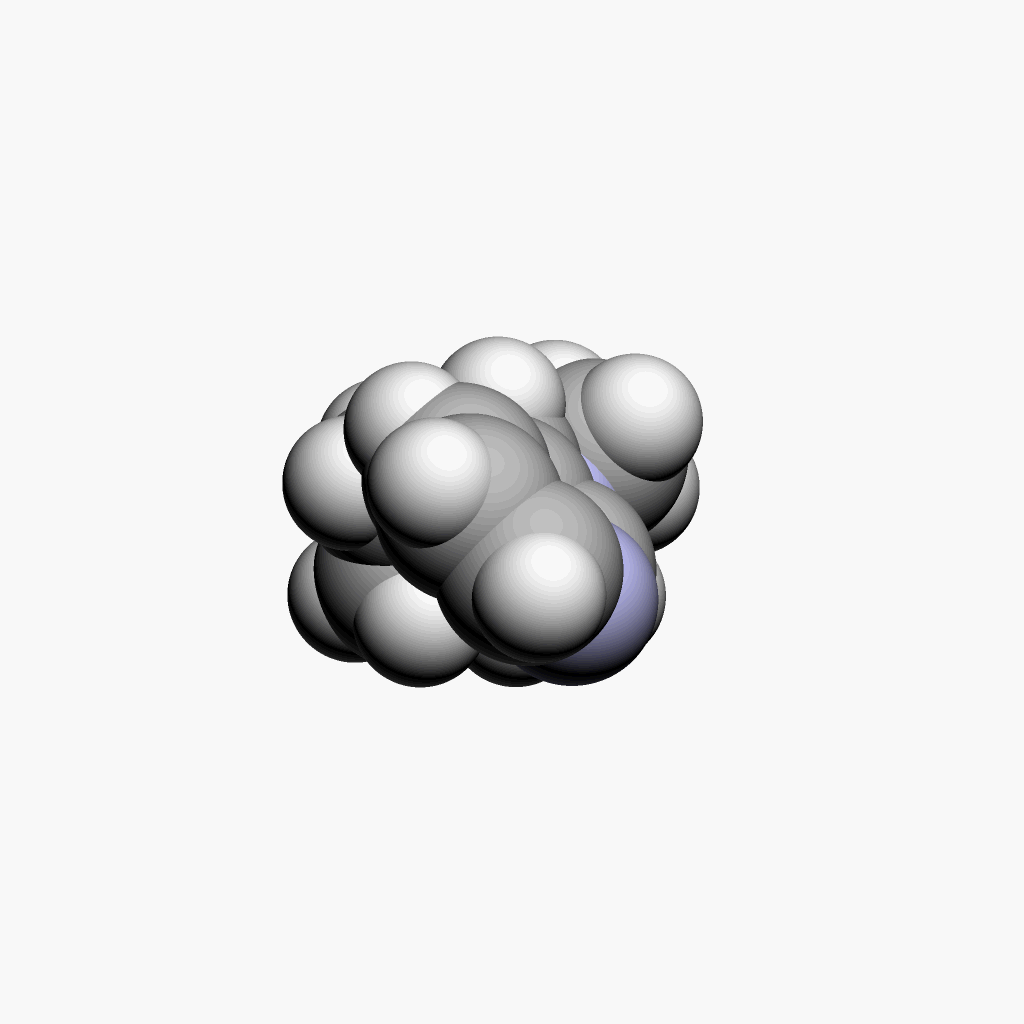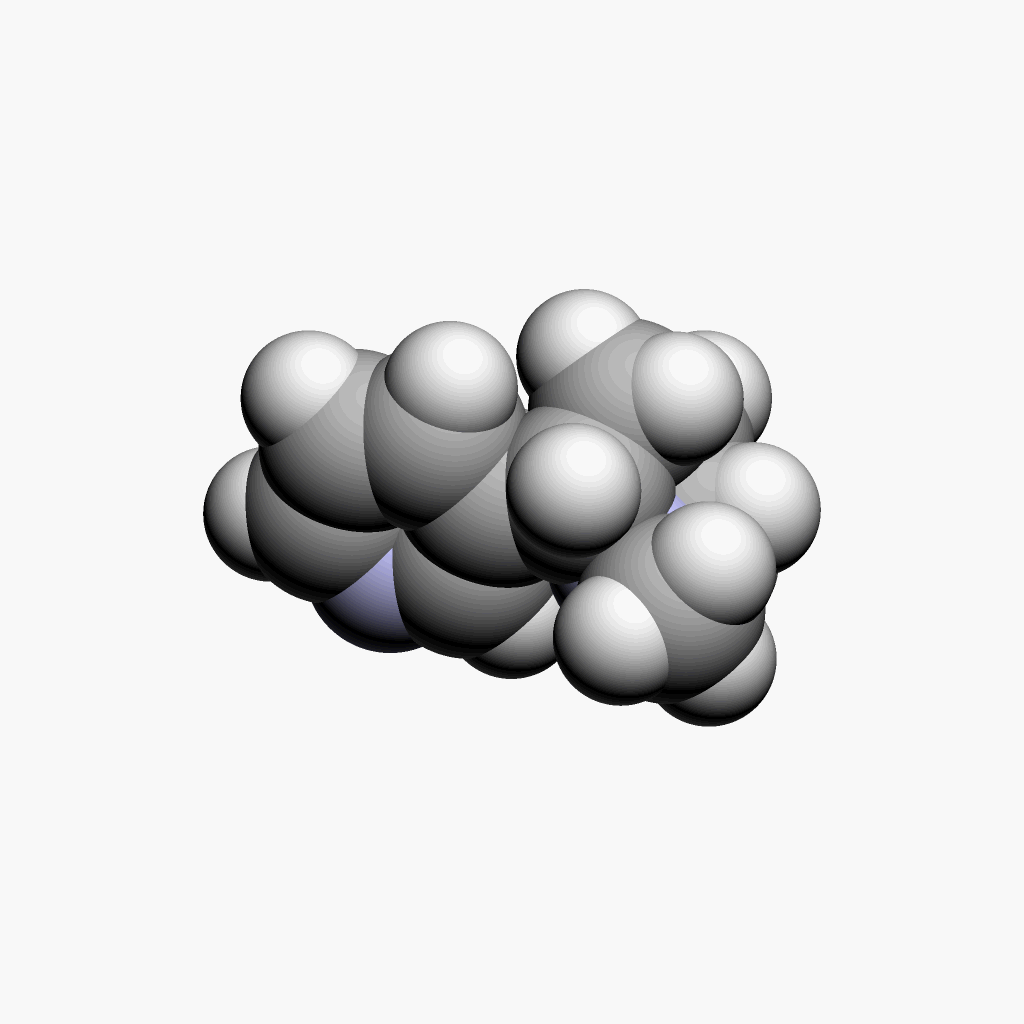 |
| Nicotine | Animated spacefill model |
One reaction that high-school students might encounter is the electrophilic substitution reaction. A common example would be where a benzene ring is attacked by an electrophile, such as Cl+, resulting in an H+ being substituted and released.
Pyridine can also undergo electrophilic substitution and it usually occur at carbon-3. Carbon-3 is the most electron-rich atom in the pyridine molecule therefore it is most susceptible to electrophilic substitution. The mechanism is shown below.
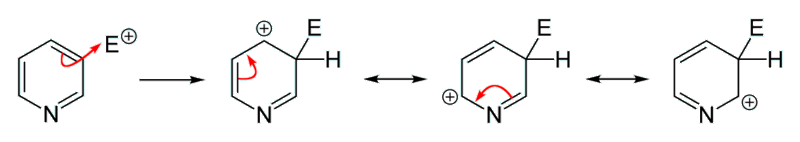
Electrophilic Substitution at carbon-3.
[Image: Ben Mills, Public domain, via Wikimedia Commons]
Pyridine is especially useful in neutralising acidic by-products as well because the molecule is much larger than ammonia so is too bulky to complete as a nucleophile. Pyridine is often described as a ‘non-nucleophilic base’. An even more effective example is the analogous molecule 2,6-di-tert-butylpyridine (shown below). The added alkyl chains make this molecule an extremely poor nucleophile. This effect is often described as ‘steric hindrance’.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.29421269]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.29421269]