![]()
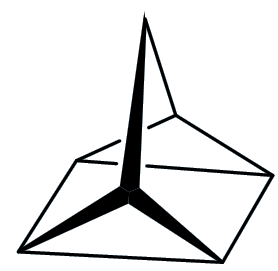
Quadricyclane
Liquid Sunshine!
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month May 2020
Also available: HTML version.
![]()


|
QuadricyclaneLiquid Sunshine!
Simon Cotton
Molecule of the Month May 2020
|
 |
It’s a bit of a shorthand. We’re talking about a chemical that happens to be a liquid, which can be used as a kind of store for solar energy.
Well, that’s a word that can be misunderstood. Fuels are something that gets burned to produce energy, and that is not the case here.
Well, let me tell you something about quadricyclane first. It is a colourless liquid that boils at 98°C (just below the boiling point of water) and has a density of 0.982 g/cm3, so it has virtually the same density as water. But here the resemblance ends.
It contains two three-membered rings (C-C-C bond angles ~60°), a four-membered ring (bond angle 90°) and two five membered rings. The normal C-C-C bond angle in saturated hydrocarbons is 109½°, so that means that the structure is under a good deal of strain – this strain energy has been calculated to be ~400 kJ/mol.
You would think so, but there seems to be a big energy barrier in the way of it decomposing, which brings us to its potential application.
You start with norbornadiene, another hydrocarbon which is a much more stable molecule than quadricyclane (and which is an isomer of quadricyclane). If you irradiate it with ultraviolet light, it is converted in good yield into quadricyclane. This reaction works best in the presence of ‘sensitisers’ like acetophenone.
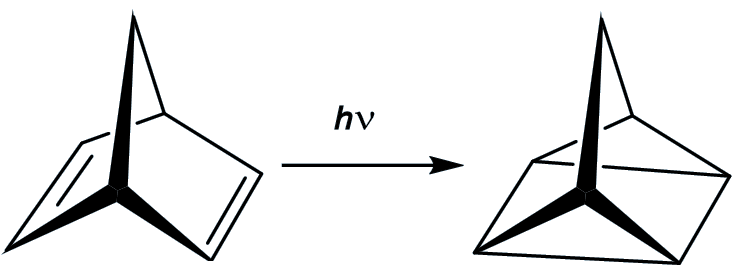
The plan is that the energy is absorbed when the norbornadiene is converted into quadricyclane, and thus stored in this ‘energy rich’ isomer. Then when the quadricyclane comes into contact with suitable catalysts, then it converts back into norbornadiene and releases quite a bit of energy (up to 96 kJ/mol). However, there are disadvantages.
For a start, norbornadiene only absorbs UV light – it cannot take advantage of ‘free’ visible light.
To enable it to absorb visible light, the absorption of the norbornadiene system needs moving more into the visible region (‘red-shifting’) which requires tweaking the molecule with electron-donating substituents (or one electron-donating group at one end of the double bond and an electron-attracting one at the other end). And this has to be done without forming a less stable quadricyclane derivative, and with a good quantum yield.
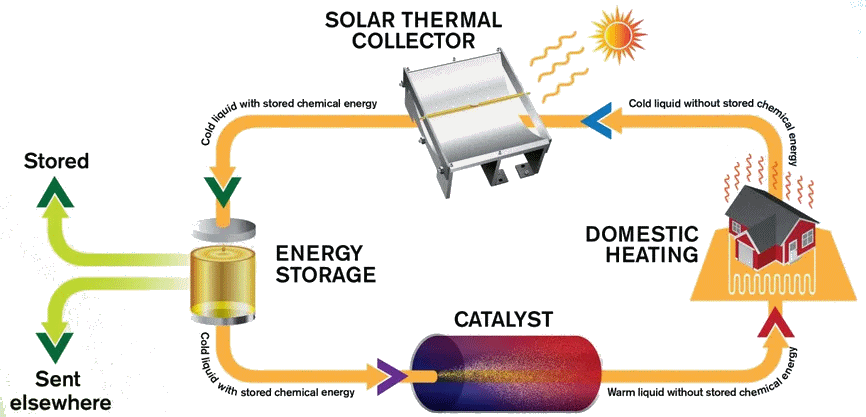
The MOST energy storage system
Image: Chalmers University of Technology
Indeed, in a system called MOlecular Solar Thermal energy storage (MOST). The modified norbornadiene (“NBD1”) has two substituents; it uses the electron withdrawing CN and the electron-donating methoxyphenyl at the opposite ends of one of its double bonds. When it absorbs light energy it forms a quadricyclane derivative (“QC1”) which has a half-life of 30 days in toluene solution.
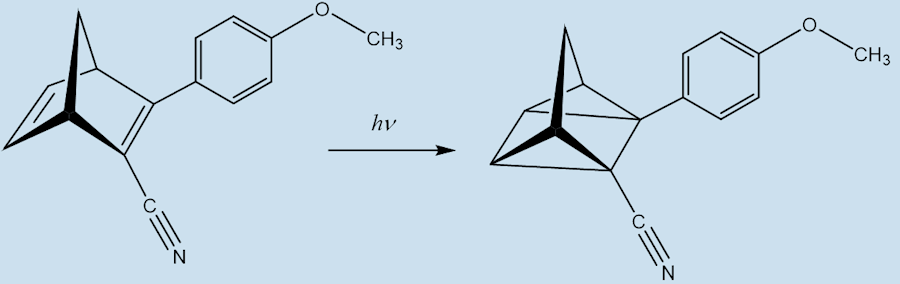
NBD1 QC1
When QC1 is passed through a cobalt phthalocyanine catalyst, on an activated carbon support, it converts back to NBD1 and releases a good deal of heat – the temperature of the fuel rises by up to 63°C.
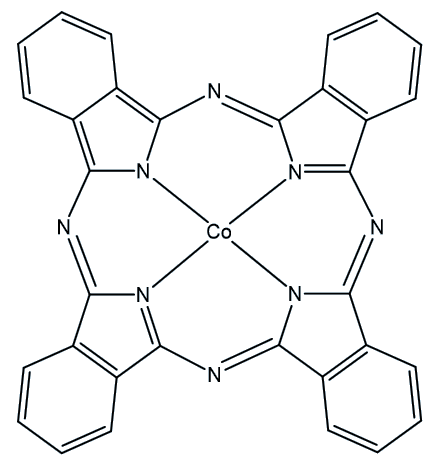
Cobalt phthalocyanine catalyst
This area is very much ‘under development’ and a great deal needs to be done before a feasible system for practical use is put forward. New factors keep being discovered, like the role of the solvent used in the system - it seems that the more polar the solvent, the longer energy can be stored. However, the choice of solvent can have an even greater effect upon the quantum yield, with toluene greatly favoured over more polar solvents.
Watch this space...
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8851403]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8851403]