![]()
Raspberry Ketone
or Rheosmin or Frambinone
(the smell of raspberries)
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month May 2012
Also available: JSMol version.
![]()

|
Raspberry Ketoneor Rheosmin or Frambinone(the smell of raspberries)
Simon Cotton
Molecule of the Month May 2012
|
 |
The smell of raspberries is due to lots of molecules, but raspberry ketone is the "impact molecule" associated with their particular smell. It's also found in other fruits, including cranberries and blackberries.
I'm not sure about Rheosmin, but Frambinone is evidently derived from framboise, the French word for raspberry. And it is simpler than the systematic IUPAC name, 4-(4-hydroxyphenyl)butan-2-one.
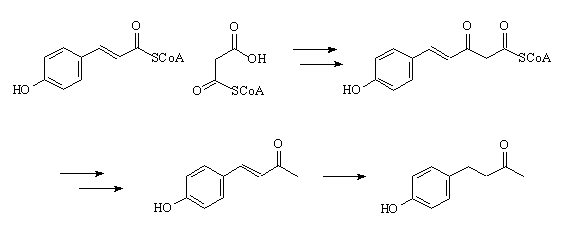
 How do plants make it?
How do plants make it?It's a multi-stage reaction that starts with a condensation of p-Coumaroyl-CoA with Malonyl-CoA.
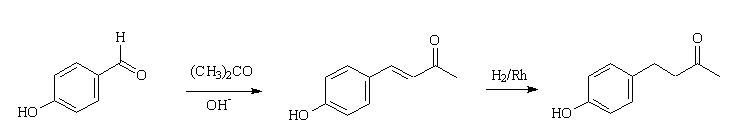
It can be made in the laboratory by more than one route. One convenient two-step synthesis involves, first, the crossed-aldol condensation of 4-hydroxybenzaldehyde with propanone, forming (4-(4'-hydroxyphenyl)-3-buten-2-one). This double-bond in the side-chain can then be catalytically hydrogenated forming rheosmin.
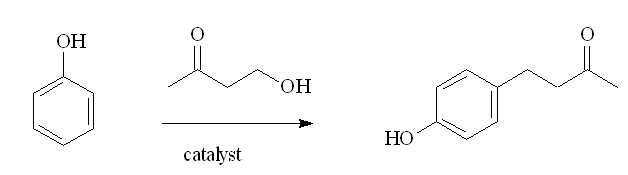
Another method that has been described involves a Friedel-Crafts alkylation of phenol by 4-hydroxybutan-2-one, using a cation-exchanged montmorillonite catalyst
Fruits give out complicated mixtures of organic molecules. Over 200 molecules have been identified in raspberry flavour. Whilst strawberry emissions are dominated by esters, up to 90% in some cultivars, terpenoids, ketones and aldehydes make up the majority of emissions from raspberries, though there are variations from one cultivar to another.
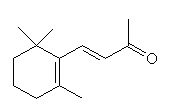
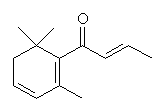
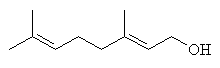
Among the important compounds with smells characteristic of ripe fruit are α- and β-ionone, β-damascenone, linalool and geraniol.
 |
 |
 |
α-ionone |
β-ionone |
β-damascenone |
 |
 |
|
geraniol |
linalool |
It is important to the flavour industry, and "natural" raspberry ketone commands a premium, so scientists have devised methods to check on the biosynthetic origin of these molecules. It can be done using 12C:13C isotope ratios, which depend upon the biosynthetic pathway, but site-specific natural abundance 2H NMR is also used. It is also possible to monitor other molecules present, like the ionones, using gas chromatography-isotope ratio mass spectrometry in the combustion and pyrolysis modes (HRGC-C/P-IRMS)
 Such an attractive smell!
Such an attractive smell! And irresistible, if you happen to be a melon fly (Dacus cucurbitae, picture, right). These are found across a wide area of Southeast Asia (China, Japan, Thailand, the Philippines, New Guinea and northern Australia) and are strongly attracted by raspberry ketone. Raspberry ketone has been found in the rectal glands of the Queensland fruit fly, Bactrocera tryoni Froggatt.
And irresistible, if you happen to be a melon fly (Dacus cucurbitae, picture, right). These are found across a wide area of Southeast Asia (China, Japan, Thailand, the Philippines, New Guinea and northern Australia) and are strongly attracted by raspberry ketone. Raspberry ketone has been found in the rectal glands of the Queensland fruit fly, Bactrocera tryoni Froggatt.
The Bulbophyllum apertum orchid flower (pic, left) has raspberry ketone in its nectar and attracts males of several fruit fly species belonging to the genus Bactrocera, not just Dacus cucurbitae but also B. albistragata, B. caudatus and B. Tau, and is rewarded through pollination by these insects.
Tests on mice indicate that it has an antiobese effect, believed to be due to its increasing lipolysis and fatty acid oxidation. As far as is known, no tests on humans have been reported, but this has not stopped a "raspberry ketone" industry from growing up.

![]()
Chapman and Hall Combined Chemical Dictionary compound code number: GZX39-H.

![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255665]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255665]