
![]()
Retinal
The molecule of sight
![]()
Joshua Howgego
and Paul May,
Bristol University, UK
![]()
Molecule of the Month April 2009
Also available: HTML version.
![]()

 |
RetinalThe molecule of sight
Joshua Howgego
Molecule of the Month April 2009
|
 |
Retinal is an interesting molecule because it is the reason we are able to see. Or at least one of them. Retinal, or more correctly, 11-cis-retinal, is a small molecule which fits into the binding site of a large protein called opsin. Together they make up rhodopsin (also known as 'visual purple', the structure of which is shown below. This is where the terms 'rods' (think Rhodopsin) and 'cones' come from, referring to cells in the retina of our eyes which contain rhodosin and isodoposin pigments, respectively.
 |
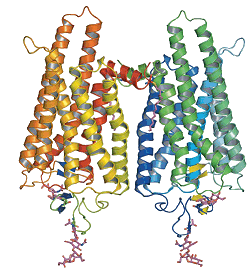 |
| A spacefill structure of cis-retinal | The native form of opsin from bovine rod cells. The pink bit at the bottom is retinal. |
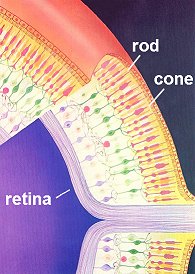 Rods and Cones
Rods and ConesHave you ever noticed when you're in bed and can't sleep that once your eyes have become accustomed to the gloom, you can actually see the outlines of items in the room quite clearly? They all look grey and colourless, but the reason we can see these outlines at all is because our rod cells are sensitive to very low levels of light. They can only respond to it in a black and white fashion though, which is why everything looks grey. The cone cells, which respond to the whole spectrum of colours, require a much higher threshold of light than is present in the dark room to be triggered.
So what makes Rhodopsin special as a molecule - how is it that it allows us to see? Firstly, it's important to realise that there is, of course, not just one, but lots of rod cells (about 100 million) coating the retina of our eyes. Each one can only ever be on or off, but when we consider all of the signals in unison we can see a picture - a bit like pixels in a digital camera. The diagram on the right shows the rods (thin) and cones (more thickly-formed) as pink forms embedded in the anterior of the retina's surface.
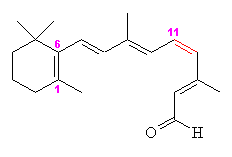
Retinal comes in two forms, 11-cis- and all-trans. The -cis prefix comes from the fact that one of the double bonds (at the 11th carbon) has the two largest substituents (that is, the largest chains coming off it) on the same side. The other double bonds are all -trans, or with the bulky substituents positioned on opposite sides. A more modern nomenclature uses the letters E (from the German, entgegen; apart) and Z (from zusammen; together). The trans- (E) version is long and straight, whereas the cis- (Z) version is bent in two.
 |
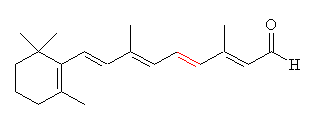 |
||
| 11-cis-retinal | All-trans-retinal |
Retinal itself is what chemists refer to as 'delocalised' - hopefully the graphic below will give you an idea of what we mean by this; the electrons contained in the delocalised system exist in a high energy 'cloud' above and below the plane of the structure. It is this extensive delocalisation of the electrons that allow retinal to absorb light so strongly.
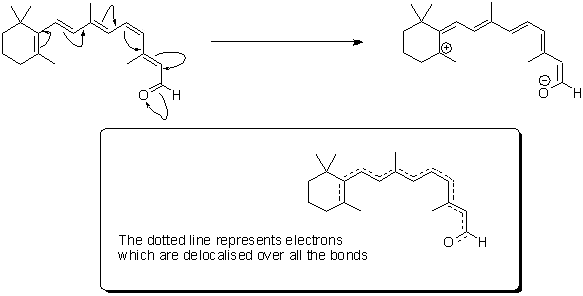
'Resonance structures' of retinal - the high energy electrons in the double bonds can flow quickly in the course shown by the arrows.
In real-time, retinal exists as a 'resonance hybrid' (bottom) of the left- and right-hand structures.

 Opsin does not absorb visible light, but when it is bonded with 11-cis-retinal to form rhodopsin, the new molecule has a very broad absorption band in the visible region of the spectrum. The peak of the absorption is around 500 nm, which matches the output of the sun closely. When a photon of light falls onto rhodopsin, the molecule absorbs the energy and the cis-double-bond between C-11 and C-12 in the retinal is temporarily converted into a single bond. This means the molecule can now rotate around this bond, which it does by swivelling through 180°. The double bond then reforms and locks the molecule back into position in a trans configuration. Thus, the light has isomerised the molecule from cis to trans, and as it did so, it changed the shape of the retinal from curved to straight. Essentially, the energy in a photon has been converted into atomic motion.
Opsin does not absorb visible light, but when it is bonded with 11-cis-retinal to form rhodopsin, the new molecule has a very broad absorption band in the visible region of the spectrum. The peak of the absorption is around 500 nm, which matches the output of the sun closely. When a photon of light falls onto rhodopsin, the molecule absorbs the energy and the cis-double-bond between C-11 and C-12 in the retinal is temporarily converted into a single bond. This means the molecule can now rotate around this bond, which it does by swivelling through 180°. The double bond then reforms and locks the molecule back into position in a trans configuration. Thus, the light has isomerised the molecule from cis to trans, and as it did so, it changed the shape of the retinal from curved to straight. Essentially, the energy in a photon has been converted into atomic motion.
Whereas the 11-cis-retinal fitted into the opsin binding-site perfectly, all-trans-retinal is the wrong shape. The linkage between retinal and opsin becomes unstable, and the molecule undergoes a series of shape changes to try and better fit the binding site, before eventually breaking free of the opsin altogether. These rapid movements of the retinal are tranfered to the protein, and from there into the lipid membrane and nerve cells to which it is attached. This generates nerve impulses which travel along the optic nerve to the brain, and we perceive them as visual signals - sight. The free all-trans-retinal is then converted back into the cis form by a series of enzyme-catalysed reactions, whereupon is reattaches to another opsin ready for the next photon to begin the process again. The way the molecule fits into opsin, which itself fits into the membrane of the disc walls, which form part of the rods, is shown below.
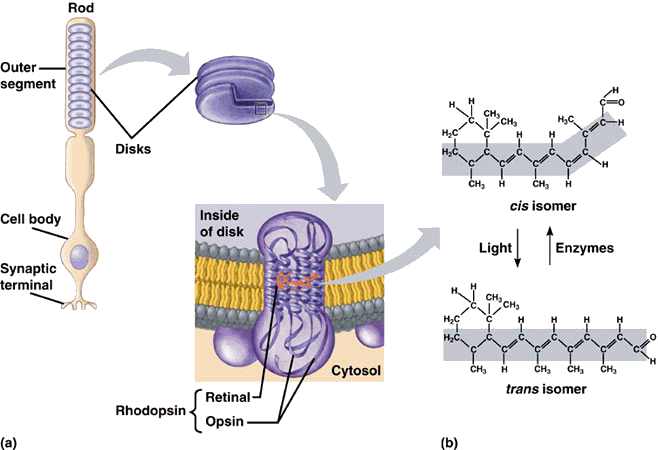
11-cis-retinal changes shape when it absorbs a photon. This causes the rhodopsin to twist, which makes the membrane it is attached to straighten out.
The membrane forms the wall of a disk, which then also straightens. Many discs moving together cause the whole rod to twitch,
sending a signal to the synaptic terminal. This signal is sent to the brain which interprets it as vision.
In order to see colour, we have 3 types of visual receptors, sensitive to red, green and blue light (situated in the cone cells), but the primary molecule involved in all of these is still 11-cis-retinal. The different wavelength sensitivities are due to small variations in sidegroups attached to the opsin protein.
Retinal is part of a group of retinoids including retinol (also known as vitamin A) and their parent molecule β-carotene. These compounds are not made in animals, but plants produce lots of them. Interestingly olives, which are distinctly not orange, also contain high concentrations of these molecules. This is where the old saying 'carrots help you see in the dark' comes from - unless humans get enough retinoids in their diets, they cease to be able to produce retinal. This can result in conditions like the scary sounding 'night blindness.' Relax though: this is hard to contract on a sensible diet.
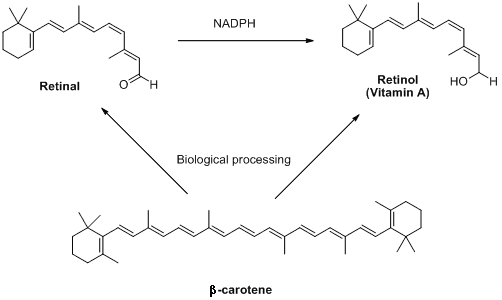
/
The retinoids retinal, retinol and β-carotene (so called because it gives carrots their intense orange colour)
are all interlinked by biosynthetic pathways.
NADPH is a biological source of negatively charged hydrogen (hydride).
But retinal is even more universal than that. There are only 3 types of eyes that can resolve images, those found in molluscs, arthropods and vertebrates. The 3 kinds of eyes are anatomically very different and probably evolved independently. However, they all utilise 11-cis-retinal as their light-receptor molecule! There are many reasons for this. Firstly, 11-cis-retinal is extremely sensitive to light, and absorbs it very strongly. Moreover, this absorbtion can be readily shifted into the visible region of the spectrum. Secondly, in the absence of light, the molecule is very stable, i.e. it does not isomerise in the dark. If this were not true, we would be constantly plagued by false images. Third, the structural change produced by isomerisation is relatively large (over 7 Å), sufficient to generate a nerve impulse. And finally, the precursors to 11-cis-retinal, the carotenes, are readily available in various edible plants.
 Retinol and skin cream?
Retinol and skin cream?You may be scratching your head at this point, thinking, 'where have I been hearing about retinol recently?' Well, since L'Oreal have recently been inundating us with reports of how wonderful Pro-retinol A, their latest miracle skin cream is, this is probably not suprising.
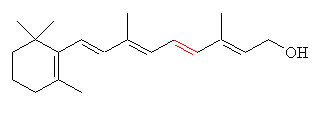 |
|
| All-trans-retinol (Vitamin A) |
These pro-retinols (can be read as precursors to retinols) break down to retinol on exposure to the skin. Vitamin A itself is what does all the work. As well as being the precursor to retinal, it is also a chemical messenger, one function of which is to instruct cells to begin multiplying more uniformly, and to produce more elastin and collagen: two protein building materials essential in healthy, young-looking skin cells.
![]()