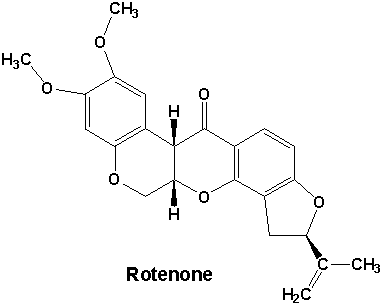
 Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia and many countries in Southern Asia. It is found in the roots and stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus spp.) and Hoary Pea (Tephrosia spp.) being the most common.
Rotenone
is a naturally occurring rotenoid plant extract from South America, Australia and many countries in Southern Asia. It is found in the roots and stems of several tropical plants, Jewel vine (Derris spp.), Lacepod (Lonchocarpus spp.) and Hoary Pea (Tephrosia spp.) being the most common.
Rotenone is generally unstable and will decompose quickly in water, sometimes as fast as two weeks after its application. However, it can sometimes persist for up to six months depending on a variety of factors including light, temperature, depth, dose and presence of organic debris. Rotenone readily breaks down in the presence of light into at least 20 products, only one of which, 6ab, 12ab-rotenolone, is toxic. None of the other 
degradation products are toxic meaning it is considered safe for use on land and in water.
The decomposition process occurs at a faster rate as the temperature of the water increases. The depth and the presence of organic debris in the water will affect the amount of light and therefore the rate of rotenone degradation, as a lack of light corresponds to a slower rate of degradation.

For centuries, South and Central American people have used the Jewel vine to stun fish. When the vine is crushed up and thrown into the water, the fish cannot inhale oxygen through their gills and come to the surface, making it possible for them to kill the fish with their bows and arrows. Also, in World War Two, Rotenone was used to kill lice in the trenches. These are the two main commercial uses of Rotenone today, as a piscicide and as an insecticide.
Rotenone is one of seven naturally occurring chemicals permitted for occasional restricted use in organic farming. It is generally used to control aphids as well as
the raspberry beetle and sawflies. However, it is rarely used as it can have an adverse effect on beneficial species as well as pests. Unlike modern, synthetic pesticides it breaks down easily and quickly and so was thought to pose little danger. It is also used as a garden dust to control insects and rotenone-based pesticides are on sale across Britain.
organic farming. It is generally used to control aphids as well as
the raspberry beetle and sawflies. However, it is rarely used as it can have an adverse effect on beneficial species as well as pests. Unlike modern, synthetic pesticides it breaks down easily and quickly and so was thought to pose little danger. It is also used as a garden dust to control insects and rotenone-based pesticides are on sale across Britain.
Rotenone is also on sale as an insecticide shampoo for dogs, cattle and sheep, which is used to kill lice.
 Another use of rotenone is to kill fish parasites. This must only be done
using very small doses, as rotenone is also toxic to fish. In October 2000, rotenone was used in a fjord in Western Norway to kill cod parasites. It was used in small enough quantities so as to kill the parasites while allowing the cod to survive. Rotenone has also been used in Norwegian rivers in attempts to eradicate the salmon parasite gyrodactylus.
Another use of rotenone is to kill fish parasites. This must only be done
using very small doses, as rotenone is also toxic to fish. In October 2000, rotenone was used in a fjord in Western Norway to kill cod parasites. It was used in small enough quantities so as to kill the parasites while allowing the cod to survive. Rotenone has also been used in Norwegian rivers in attempts to eradicate the salmon parasite gyrodactylus.
The mechanism of action of rotenone is similar for both insects and fish and it is outlined on the page describing the use of rotenone as a piscicide.
Rotenone is the most commonly used pesticide to eradicate fish populations. For many years, introduced fish species have caused desirable native species to suffer because of the unwanted species’ competitiveness. When a pond or lake has an unbalanced fish population and is therefore undesirable to recreational fishermen, a common
solution is to eradicate the fish population completely and introduce new fish in a more desirable combination.

However, as with any pesticides, rotenone must be used carefully. In California in 1997, a lake that was a primary
water source for 2,300 people was treated with rotenone in order to kill Northern Pike. The pike had been illegally introduced into the lake and were threatening to destroy prized salmon and trout fisheries. However the pesticide killed all the fish in the lake. The residents were forced to find an alternative water source, as the seven-mile lake
was still contaminated six months later.


Rotenone is able to inhibit cellular respiration in almost every living organism, including mammals, fish, amphibians, insects and even plants. So why is rotenone only toxic in this way to fish and insects? Fish are highly susceptible because rotenone can efficiently and quickly enter the blood stream through the gills. This is ompounded by the fact that rotenone is very insoluble in water and the gills have a relatively high lipid content that the rotenone will favour. The trachea of insects acts in a similar way to the gills of a fish meaning rotenone can also act quickly on insects. Mammals are not usually affected by rotenone as it is inefficiently absorbed from the gastrointestinal tract.
Rotenone poisoned fish can be revived by simply putting them into untreated fresh water. Rotenone can also be detoxified by solutions of potassium permanganate, chlorine or methylene blue. The use of methylene blue is not recommended because it increases bacterial infection on fish. Potassium permanganate is the most effective at
detoxification and is used to reverse the toxicity in the affected fish or to accelerate the natural breakdown in water. It is often used on water in outlet streams flowing out of treated lakes so that rotenone will not kill desirable fish species that live in the stream.

Rotenone treatments in municipal water supplies have often been criticised because they can cause water to have a bad odour
and taste. Adding activated carbon to the treated water can control these effects. Also, if the rotenone is applied during the summer it will degrade faster due to the increased temperature and light levels.
Parkinson’s disease is one of the most common neurodegenerative diseases, affecting about 1% of all people over the age of 65. It affects the Pope, Michael J. Fox and Muhammad Ali. The main symptoms include:
] Tremor at rest, usually starting in the hands that tends to diminish during voluntary activity,

] Muscle rigidity,
] Suppression of voluntary movements, movement is difficult to stop as well as start.
] Speech difficulties,
] Muscle weakness,
Parkinson’s sufferers tend to walk in a fast shuffle. They find it hard to start and once moving cannot quickly stop or change direction. People with Parkinson’s disease can also develop a blank, mask-like facial expression and a soft, monotonous voice. These symptoms are caused by the progressive degeneration of dopamine-containing neurones in a part of the brain called the substantia nigra. It is often associated with dementia, probably because the degenerative process also occurs in other parts of the brain. Another characteristic feature of Parkinson’s disease is that microscopic protein deposits, known as Lewy bodies are found in the brain.
Parkinson’s disease often occurs with no obvious cause, but it may be the result of pathological damage, and some cases have been linked to genetic risk factors. The main risk factor is age, but symptoms can also be drug-induced, the main drugs involved being those that either reduce the amount of dopamine in the brain or block dopamine receptors. Many antipsychotic drugs block D2 (dopamine) receptors and their main side-effect is to cause movement disorders. These disorders are reversible on stopping drug treatment. Genetically engineered mice lacking D2-receptors show greatly reduced spontaneous movement, resembling Parkinson’s disease. Unlike schizophrenia and many other neurological disorders, Parkinson’s disease shows no hereditary tendency, and therefore an environmental cause seems more likely.
One of the few benefits of smoking includes a significant reduction in  the risk of contracting Parkinson’s disease. The incidence of Parkinson’s among smokers is approximately half that of non-smokers. This effect is due to the activation of nicotine receptors, which benefits Parkinson’s disease by causing dopamine release.
the risk of contracting Parkinson’s disease. The incidence of Parkinson’s among smokers is approximately half that of non-smokers. This effect is due to the activation of nicotine receptors, which benefits Parkinson’s disease by causing dopamine release.
The understanding of Parkinson’s disease was advanced by a chance event. In 1982 a group of young drug addicts suddenly develo ped an exceptionally severe form of Parkinson’s. The cause was traced to the compound 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) which was a contaminant in a preparation used as a heroin substitute. MPTP produces a Parkinson’s disease-like state in primates by causing irreversible destruction to dopamine neurones in a particular part of the brain. This means that it is a very useful experimental tool for testing possible therapies for Parkinson’s disease.
ped an exceptionally severe form of Parkinson’s. The cause was traced to the compound 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) which was a contaminant in a preparation used as a heroin substitute. MPTP produces a Parkinson’s disease-like state in primates by causing irreversible destruction to dopamine neurones in a particular part of the brain. This means that it is a very useful experimental tool for testing possible therapies for Parkinson’s disease.
In an experiment conducted at Emory University in Atlanta, neurologists implanted osmotic pumps in 25 laboratory rats to continuously administer small doses of rotenone over a period of five weeks. The rotenone was dissolved in dimethyl sulfoxide (DMSO) and polyethylene glycol (PEG). Twelve of the 25 rats developed symptoms of Parkinson’s disease, including limb tremors and rigidity and examinations revealed that large numbers of dopamine producing cells in the rats’ brains had died or were damaged. The neurones of the substantia nigra in the brain also showed fibrous protein deposits that closely resemble Lewy bodies.
The results of this study do not, however, prove conclusively that rotenone causes Parkinson’s disease in humans because the method of administration was unnatural. The rotenone was continuously administered through the rat's jugular vein and it was mixed with DMSO and PEG. DMSO enhances tissue penetration of many chemicals.
Direct injection is the fastest way to introduce a chemical into the bloodstream, and in this study, the rats had continuously high levels of rotenone in their bloodstream. Normal exposure of humans to rotenone through use as an insecticide or piscicide would be ingestion, inhalation or through the skin and these significantly slow down the introduction of chemicals
into the bloodstream. Also, most peopl e using the chemical wear protective clothing, further minimising the risk of exposure. Generally, rotenone exposure is extremely limited due to the fact that it decomposes easily in the environment and in mammals and birds, rotenone is oxidised in the gut and excreted by the liver and kidneys, making it extremely unlikely that it would reach the substantia nigra in the brain. However, this does not mean that rotenone is safe, as it has been known to cause other
side-effects such as vomiting and convulsions and in 1986 there was a case of fatal rotenone poisoning in a three and a half year old girl.
e using the chemical wear protective clothing, further minimising the risk of exposure. Generally, rotenone exposure is extremely limited due to the fact that it decomposes easily in the environment and in mammals and birds, rotenone is oxidised in the gut and excreted by the liver and kidneys, making it extremely unlikely that it would reach the substantia nigra in the brain. However, this does not mean that rotenone is safe, as it has been known to cause other
side-effects such as vomiting and convulsions and in 1986 there was a case of fatal rotenone poisoning in a three and a half year old girl.
This research has opened up a more worrying prospect as rotenone works in a similar way to many other synthetic pesticides used widely in food production but which do not break down as easily. A classic symptom of exposure to widely used organophosphorus pesticides is muscle tremor, which is also a symptom of Parkinson's. There is therefore a possibility that chronic exposure to environmental toxins is linked to the incidence of Parkinson's disease and other degenerative diseases.
However, the real benefit of this study is that a new model of Parkinson's disease has been found. MPTP, as mentioned before, only induced Parkinson's-like symptoms in primates whereas rotenone administered in this manner should induce these symptoms in all mammals. This new model gives researchers a better method to study the cellular defects associated with Parkinson's disease and test potential treatments.
Outlined below are some other organically derived chemicals which are used as insecticides:
are used as insecticides:
Pyrethrin is a natural product extracted from white Chrysanthemum flowers, that have been used as insecticides for over a century. It works against a very wide range of insect pests. The main active principles of Pyrethrum flowers are known as Pyrethrins. Pyrethrin is a fast-acting poison which disrupts the nervous system and causes paralysis of the insect, while at the same time being non-toxic to warm-blooded animals. Pyrethrins are also biodegradable and they break down very quickly in sunlight, moisture and
oxygen. Pyrethrins  are usually combined with other insecticides like rotenone to ensure their effectiveness. In some people, pyrethrin can cause a skin rash, and breathing the dust can cause headaches and sickness.
are usually combined with other insecticides like rotenone to ensure their effectiveness. In some people, pyrethrin can cause a skin rash, and breathing the dust can cause headaches and sickness.
Annona trees are grown for fruit in Vietnam. The most useful for making botanical insecticides is custard apple or binh bat (Annona muricata). The botanical insecticide is contained in the unripe fruits, leaves, roots, and especially seeds.
It works as a stomach poison, contact poison and repellant. It is said to be effective against leafhoppers and caterpillars.
making botanical insecticides is custard apple or binh bat (Annona muricata). The botanical insecticide is contained in the unripe fruits, leaves, roots, and especially seeds.
It works as a stomach poison, contact poison and repellant. It is said to be effective against leafhoppers and caterpillars.
 Neem, derived from the neem tree (Azadiracta indica) of semi-arid tropical regions, contains many active compounds that act as feeding deterrents and as insect growth regulators. The main active ingredient is azadiractin, which is said to be effective on 200 types of insects, mites and nematodes. These include caterpillars, thrips and whiteflies. It has low toxicity to mammals, including
people. It is also biodegradable and loses its effectiveness when exposed to direct sunlight.
Neem, derived from the neem tree (Azadiracta indica) of semi-arid tropical regions, contains many active compounds that act as feeding deterrents and as insect growth regulators. The main active ingredient is azadiractin, which is said to be effective on 200 types of insects, mites and nematodes. These include caterpillars, thrips and whiteflies. It has low toxicity to mammals, including
people. It is also biodegradable and loses its effectiveness when exposed to direct sunlight.
Nicotine, derived from tobacco, is extremely toxic and fast acting on most animals. The nicotine of half a cigarette, if injected into the blood, is enough to kill a full-grown man. An additional danger of using tobacco leaf
extract is that this extract may contain a virus disease called Tobacco Mosaic Virus, or TMV. This virus disease affects a wide range of plants, for example tomato and aubergine. Nicotine kills insects by contact, and if inhaled or eaten. The most common use is to control soft-bodied insects such as aphids, mites, and caterpillars.
blood, is enough to kill a full-grown man. An additional danger of using tobacco leaf
extract is that this extract may contain a virus disease called Tobacco Mosaic Virus, or TMV. This virus disease affects a wide range of plants, for example tomato and aubergine. Nicotine kills insects by contact, and if inhaled or eaten. The most common use is to control soft-bodied insects such as aphids, mites, and caterpillars.
Marigolds are often grown in gardens for their attractive flowers, and are even cultivated commercially for use as cut flowers. But marigold can also have a repellant effect on insects and nematodes. Dried marigold when incorporated into soil has been found to improve the overall health of young plants. Fresh marigold tea also repels caterpillars on cabbage, but only for a few hours.
Chilli, or chillipepper. The ripe fruits and see d contain insecticidal compounds and they can control aphids and caterpillars. Highly concentrated chili solution has been known to produce similar results to synthetic insecticides.
d contain insecticidal compounds and they can control aphids and caterpillars. Highly concentrated chili solution has been known to produce similar results to synthetic insecticides.
Links
Listed below are links to the websites I used during my research:
http://www.adoniss-extraction.com/pages/rotenone/general.htm#
http://www.pyrethrum-pages.com/Pyretrhum-links/pyretrhum-links.html
http://members.tripod.com/~ngin/JM092.htm
http://www.nature.com/neuro/journal/v3/n12/pdf/nn1200_1301.pdf
http://www.nature.com/neuro/press_release/parkinsons.html
http://www.pan-uk.org/press/parkinso.htm
http://www.nature.com/nsu/001109/001109-4.html
http://more.abcnews.go.com/sections/living/dailynews/parkinsons001106.html
http://ace.ace.orst.edu/info/extoxnet/pips/rotenone.htm
http://msucares.com/pubs/pub1954.htm
http://s.o.w.tripod.com/rotenone.htm
http://www.lockepet.com/itm00669.htm
http://www.pulseplanet.com/archive/Nov99/2010.html
http://www.astm.org/JOURNALS/FORENSIC/PAGES/788.htm
http://www.ets.uidaho.edu/etox_fall02/resources/Case%20Study/ROTENON2.PDF
http://www.fisheries.org/rotenone/
http://www.awlonline.com/mathews/ch15/rotenone.htm
Reference
Pharmacology, 4th Edition, H.P. Rang, M.M. Dale and J.M. Ritter, Churchill Livingstone.
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5371486]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5371486]