![]()
Silane
Silicon's equivalent of methane
![]()
Oulenda Wu and Stephen Belding
Rugby School, UK
![]()
Molecule of the Month January 2025
Also available: HTML version.
![]()

A fire from a leaking silane cylinder
SilaneSilicon's equivalent of methane
Oulenda Wu and Stephen Belding
Molecule of the Month January 2025
|
 A fire from a leaking silane cylinder |
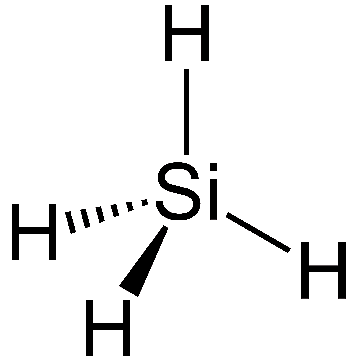
Silane has a chemical formula of SiH4 and is a compound composed only of silicon and hydrogen atoms. It forms a tetrahedral structure and is the simplest stable silicon hydride. In this way it resembles methane, CH4, which is the simplest stable carbon hydride.
 |
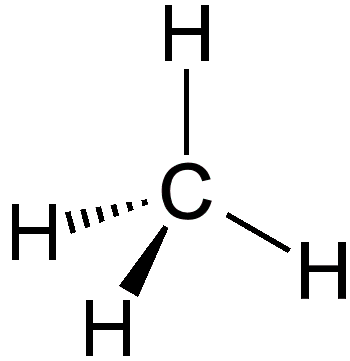 |
| Chemical Structure of silane | Chemical Structure of methane |
Silane is a colourless gas with a sharp, repulsive odour. It is explosively flammable in air, as you can see from the picture at the top of the page of a leaking silane cylinder! It holds practical significance as a precursor to elemental silicon, with a variety of uses across industrial and commercial sectors.
Two prominent German chemists, Friedrich Wöhler and Heinrich Buff, first discovered silane in the early 1850s. Friedrich Wöhler was renowned for his groundbreaking synthesis of urea (MOTM for June 1996), the first organic compound made from inorganic materials. By reacting silicon tetrachloride with molten lithium aluminium hydride, they produced pyrophoric silane gas. This reaction was significant as it demonstrated that silicon, like carbon, could form stable compounds with hydrogen, laying the foundation for silicon-based chemistry.
The reaction for the initial formation of silane gas:
SiCl4(l) + LiAlH4(l)  LiCl(s) + AlCl3(l) + SiH4(g)
LiCl(s) + AlCl3(l) + SiH4(g)
 |
 |
| Friedrich Wöhler [Image: Engraving by C. Becker after Fritz L‘Allemand, CC BY 4.0 via Wikimedia Commons] |
Heinrich Buff [Image: Public Domain, Wikimedia Commons] |
Highly reactive, flammable and dangerous, why?Silane has an Si-H bond length of approximately 1.48 Å, which is slightly longer than the C-H bond length in methane (1.09 Å). The larger size of silicon atoms compared to hydrogen results in weaker bonds with lower electron density. This lower bond energy contributes to silane's high reactivity, particularly in its reaction with oxygen. Silane ignites spontaneously upon contact with air, without the need for an ignition source like a spark, hot surface, or open flame! The property is termed ‘pyrophoric’. This exothermic reaction produces silicon dioxide and water, releasing a significant amount of heat. Inhalation of silane can cause severe respiratory irritation. The combustion reaction is: SiH4(g) + 2O2(g) |
 Silane combusting in air [Image: Михаэльс, CC BY 4.0 via Wikimedia Commons] |
There are several routes to producing silane. One laboratory-scale method involves the hydrolysis of magnesium silicide (Mg2Si) in hydrochloric acid (HCl), starting from silicon dioxide (sand) and magnesium. The process follows these reactions:
 2MgO(s) + Si(s)
2MgO(s) + Si(s) Mg2Si(s)
Mg2Si(s)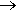 SiH4(g) + 2MgCl2(s)
SiH4(g) + 2MgCl2(s)The product, silane, spontaneously ignites on contact with oxygen in air. This is a common lecture demonstration, as can be seen in this video. This process generates a significant array of byproducts, including Si2H6, Si3H8, and Si4H10, along with traces of higher silanes. Due to the reaction's lack of specificity, it holds limited practical value outside of lecture demonstrations.
Silane plays a crucial role in semiconductor manufacturing, where it is used to produce ultrapure silicon. This silicon is typically 99.9999999% pure (9N, for nine nines of purity) and can reach 99.999999999% purity (11N) for advanced applications. Ultrapure silicon is essential for creating integrated circuits and other electronic components, as even trace impurities can significantly impact semiconductor performance.
Silane also has a very important use to make hydrogenated amorphous silicon (a-Si:H), which is a vital component in all the photovoltaic cells used for solar power generation.
 |
 |
| Silicon wafers [Image: Saparaud, CC BY-SA 3.0 via Wikimedia Commons] |
Silicon-based photovoltaic solar cells [Image: Chmee2, CC BY-SA 3.0 via Wikimedia Commons] |
It can also be used as a water repellent.
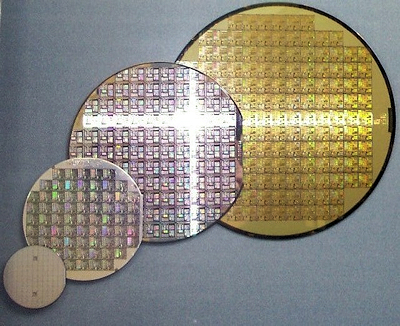
Ah, we don't use pure silane but derivatives of it called alkyl-modified silanes. These are not water-soluble and have low interaction with inorganic surfaces. However, hydrolysis of these silanes increases their interaction through hydrogen bonding, and some molecules even polymerise. Once dried, these materials exhibit strong water repellency. Concrete, mortar, and masonry are building materials with porous and capillary structures that readily absorb water. To protect these materials from water damage, water-repellent silane-based sealants are used to minimize their absorbency. Organo-silicon compounds are often called silicones, and are well known for applications as containers in food and dairy processing, silicone rubber sealants in manufacturing, cosmetics, car airbags, as electrical insulators and in medical components, such as breast implants.
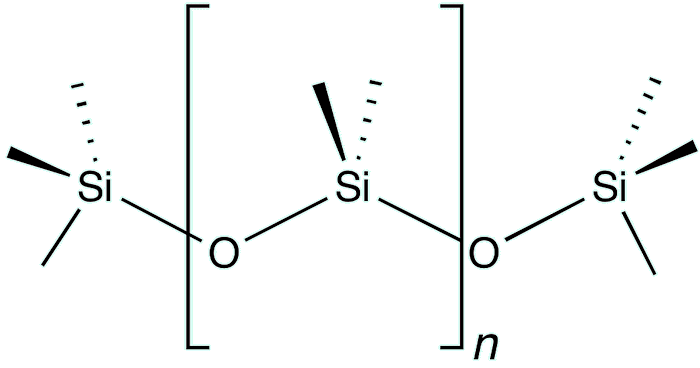
Polydimethylsiloxane (PDMS) is the principal component of silicones.

Applications of silicones. (a) Silicone sealant. (b) Silicone insulating tape. (c) Silicone insulated wires. (d) Silicone moulds for cooking and baking. (e) Silicone breast implant.
Is there a silane equivalent for ethane?Yes indeed! Si2H6 can be produced in high yield by irradiating SiH4 using a CO2 laser. Silanes, represented as SinH2n+2, with straight or branched chains, have been well characterised up to n = 10. These molecules form a homologous series analogous to the alkanes. However, due to the relative weakness of the silicon-hydrogen bonds, silanes are limited in size. |
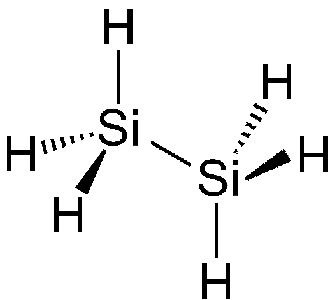 Chemical Structure of disilane |
The graph below shows the melting and boiling points of the first 10 straight-chain alkanes and silanes. All lines exhibit a smooth trend with increasing chain length, reflecting stronger van der Waals’ forces. The silanes melt and boil at higher temperatures than the corresponding alkanes because silicon atoms are larger than carbon, leading to stronger temporary and induced dipoles, which, in turn, result in enhanced van der Waals’ forces.
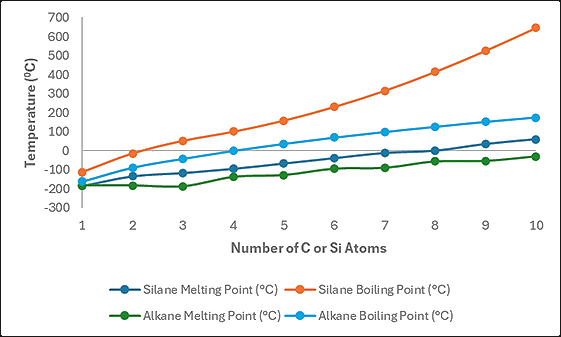
The melting and boiling points of the first 10 straight-chain alkanes and silanes.
Investigations into the formation mechanisms of higher silanes in 2016 suggested synthesis was possible up to nonadecasilane (Si19H40). However, the production of longer-chain silanes poses significant challenges due to their high reactivity and instability. These characteristics limit the exploration of the properties and potential applications of higher silanes.
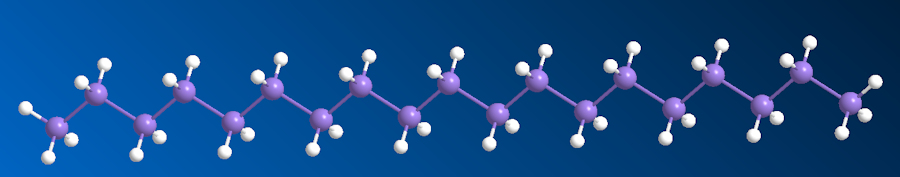 |
|
| Nonadecasilane |
A homologous series known as germanes (GenH2n+2) can be formed, which includes straight and branched chains extending up to n = 9. Germane (GeH4), a colourless gas, is less reactive than silane (SiH4) and can be synthesised by reacting germanium dioxide (GeO2) with sodium borohydride (NaBH4), although this process also yields higher germanes as by-products. When tin(IV) chloride (SnCl4) reacts with lithium aluminium hydride (LiAlH4), it generates stannane (SnH4), which decomposes slowly at room temperature. The reactivity trend among these compounds is as follows: SiH4 > GeH4 < SnH4. Plumbane (PbH4) can be synthesised from lead(II) nitrate (Pb(NO3)2) and NaBH4; however, it decomposes in less than 10 seconds at room temperature. As anticipated, the infrared spectrum of PbH4 demonstrates a tetrahedral structure, showcasing the predictive power of the periodic table!
 |
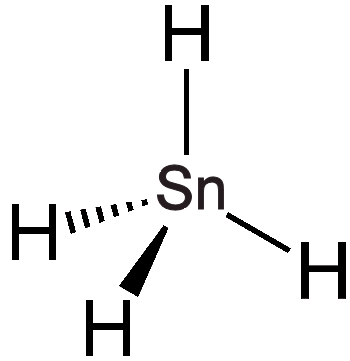 |
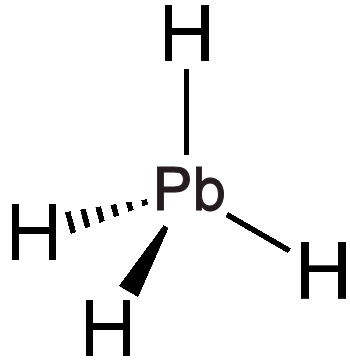 |
| Chemical Structure of germane | Chemical Structure of stannane | Chemical Structure of plumbane |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27377652]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27377652]