![]()
Sodium Chloride
Table salt
![]()
Sophie Goodall
University of Bristol
![]()
Molecule of the Month - June 2024
![]()

|
Sodium ChlorideTable salt
Sophie Goodall
Molecule of the Month - June 2024
|
 |
 So, sodium chloride is salt?
So, sodium chloride is salt?Yes, it is! Sodium chloride goes by a few names, ‘salt’, ‘common salt’, ‘table salt’ or ‘halite’. It has the chemical formula NaCl, with the name quite simply coming from the the 1:1 ratio of sodium and chloride ions present in the ionic compound. There are language variations of the word salt. The word is derived from the Proto-Indo-European root "sal-," meaning "to shine" or "to glitter". This root is the source of the Latin word sal which translates to ‘salt’. It has been an important part of world history dating back to 6050 BC, and while a weird notion today as it is so commonplace, in ancient times salt was highly valued, with legal restrictions placed upon its production, wars fought over it, and fortunes made from its trade. It was of great economic importance, with not just for it's taste but mainly for its role in preserving food over the winter.
That is a common idea, which came about from the latin word salarium (salt money) from which we get the modern word 'salary'. But modern historians can find no evidence that this is actually true - Roman soldiers were paid in coins like everyone else. So how did the myth come about? First, the Romans imposed taxes on salt production and sales, which perhaps led to confusion with soldiers' pay. Also, because of its cultural significance and importance for preserving food, the word salarium may have become confused with wages. But one Latin-based word which we still use correctly is ‘salad’ originating from the word ‘salt’ because the Romans always sprinkled salt on their vegetables.
It was a combination of two scientists who discovered sodium chloride. Credit for salt's discovery is universally given to Sir Humphry Davy, a British chemist and inventor, as both elements making up sodium chloride are linked to him. He was the first to prepare sodium (Na) in the laboratory in 1807 by using electrolysis to remove it from sodium hydroxide (NaOH). From its extraction, Davy found sodium was a metal. Then in 1811, he named chlorine after recognising its elemental nature. Before this, chlorine was thought to be a mixture with oxygen. Previously, in 1736, French physician Henri-Louis Duhamel de Monceau had discovered that mixing sodium carbonate and hydrochloric acid produced a solution with a salty taste. (This was before the days of health-and-safety when scientists would taste the products of their experiments!). Duhamel’s work was then completed in 1807 by Davy, who found that the ‘common salt’ produced by burning sodium metal in a chlorine vessel was chemically identical to salt.
 |
 |
| Sir Humphry Davy in 1849 [Image: Science History Institute, Public domain, via Wikimedia Commons] |
Henri-Louis Duhamel de Monceau [Image: Pierre-Etienne Moitte, Public domain, via Wikimedia Commons] |
What does it look like?Sodium chloride is a colourless/white crystalline solid, exhibiting no odour. It is a water-soluble compound known as saline solution when it’s in its aqueous form. Normal saline solutions contain 9 g of sodium chloride per litre (0.9%) which is close to the sodium concentrations found in blood and tears. How is sodium chloride made?Sodium chloride is produced through a neutralisation reaction. When the base sodium hydroxide (NaOH), reacts with hydrochloric acid (HCl), it produces sodium chloride as a salt and liquid water (H2O). NaOH(aq.) + HCl(aq.) On an atomic level the reaction proceeds by the addition of solid sodium with gaseous chlorine. 2Na(s) + Cl2(g) |
 [Image: mkupiec7 via free Pixabay licence] |
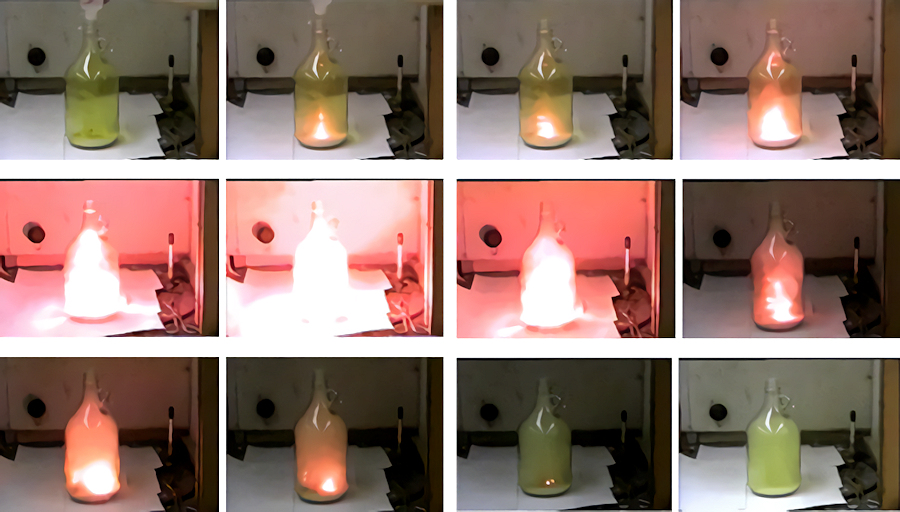
Reaction of sodium metal with chlorine gas to make salt.
From left to right. A bottle filled with chlorine gas. Metallic sodium is then placed in the flask and water is added, which reacts with the sodium making it hot.
The hot sodium then reacts with chlorine gas creating a bright yellow light, releasing heat, and white fumes of sodium chloride.
[Image: Angelo State University]
This reaction is so readily accessible as sodium is in Group 1 of the Periodic Table and therefore has one electron in its valence (outermost) shell. Sodium’s nucleus has a relatively small effective positive charge, meaning the outer electron is readily lost. Conversely, chlorine can be found in Group 17 which means it has 7 electrons in its valence shell. Chlorine also has an additional six protons in the nucleus compared to sodium, so can readily accept electrons. To form a complete valence shell and therefore be more electrically stable, the one electron from sodium is transferred to chlorine, meaning chlorine now has a full octet. This forms a sodium cation (Na+) and a chloride anion (Cl-). The reaction is highly exothermic, producing lots of heat and a bright yellow light.
No, sodium chloride is an abundant naturally occurring mineral which can be extracted from seawater and found in deposits on the ground and underground. Currently, salt is manufactured by several methods, including the evaporation of seawater and brine from salt lakes and brine wells, solution-mining of underground salt deposits and underground mining of 'rock salt'. The world leader in salt production is China, with 53 million metric tons of salt produced in 2023.
Salt mines are where natural salt deposits are extracted from underground. Rock salt is the form in which the salt is usually mined, and underground mining is the second-oldest method to produce salt. The Room and Pillar method is used and is arguably the most important underground minding method used today. In this method, 45-65% of salt is removed in a checkerboard pattern leaving permanent, solid salt pillars in place for mine roof support.
Yes, around the world there are different mining regions, with some being of note. Poland is home of the Wieliczka Salt Mine. It is a popular tourist attraction including maze-like passageways, an underground lake, four chapels, and statues carved out of rock salt.
 |
 |
| Statues carved out of rock salt at the Wieliczka Salt Mine, Poland [Image: Alice65456, CC BY-SA 4.0 via Wikimedia Commons] |
Large chandeliers made out of salt which can be found all throughout the Wieliczka Salt Mine. The light from the candles reflects off the salt crystals. [Image: Cezary p, CC BY-SA 3.0 via Wikimedia Commons] |
Like all underground salt deposits, the salt walls in the Wieliczka mine originated in the sea, as this area was submerged underwater around 14-million years ago. Over geological time, the Carpathian Mountains arose in the sea’s surrounding area,and the sea dried up. Earthquakes and volcanic eruptions buried the salt deposits underground, which cracked into pieces under the pressure of the Carpathian Mountains. Further tectonic activity shifted the salt layer upwards, allowing them to be discovered . The most precious rock salt was discovered in the 16th century and mined ever since. The hardness of the rock salt allowed the miners to carve them into statues, ornaments, and even a fully working underground cathedral. The salt also contains a lot of fossils, the remains of sea creatures imprisoned by the salt as the sea dried up.
Is that the same salt that is used on the roads in winter?Yes, rock salt, also called 'road salt' is used to de-ice slippery areas in the winter. Equally, it can be used in an anti-icing approach, being spread by winter-service vehicles in anticipation of snow or ice on the roads. Rock salt is stored in 'grit bins' and works by lowering the melting point of snow and ice, hopefully to below that of the ambient temperature, such that it melts back into water. The melted snow washes off hard surfaces, such as paved roads and parking lots, as a concentrated brine solution. In the anti-icing approach, brine prevents the snow/ice bonding to the road surface. The alternative, which is de-icing, lets the snow bond to the road. Pre-wetted rock salt is then applied, which breaks the bond between the snow and the surface. Brine, however, becomes ineffective at de-icing roads below -6.5 °C to -9.5 °C. Rock salt can also be mixed with grit and is used to improve road safety. The grit improves the friction between a car’s tyres and the road. Insufficient gritting on the roads can be dangerous for vehicles and foot traffic and so is reapplied multiple times during a single day under icy conditions. |
 A road salt truck which spreads the salt over icy roads [Image: James LeWorm, CC BY-SA 4.0 via Wikimedia Commons] |
Yes, indeed, on the famous Bonneville Salt Flats Racetrack in Utah, USA!
Salt flats are areas of flat ground covered with salt and other minerals. Salt flats are found in climates when there is more water evaporation than there is precipitation, namely the desert. It forms when a pool of water, such as a lake, evaporates leaving behind minerals which come out of the solution. Over thousands of years the minerals collect on the surface. The sun’s rays are reflected, through radiation, so the minerals appear white.
 |
 |
| Death Valley's Badwater Salt Flats at Twilight [Image: Photographersnature, CC BY-SA 3.0 via Wikimedia Commons] |
Bonneville Salt Flats, Wendover, Utah [Image: Dietmar Rabich CC BY-SA 4.0 via Wikimedia Commons] |
An well-known example of a salt flat is the one that gave its name to a city in the US - Salt Lake City. In the same state, the Bonneville Salt Flat, is a densely packed salt flat which holds approximately holds 147 million tons of salt, and roughly 90% is common table salt. It is the remains of Lake Bonneville, an ice-age lake which covered much of Utah between 14,000 and 32,000 years ago. The lake was fed by melt-water from a glacier but when the glacier receded over a period of thousands of years, Lake Bonneville had no outlets to the ocean, and so the water evaporated, leaving salt minerals behind. In turn, the “fastest place on earth” was created.
Exactly. Salt flats can go on for many miles, with their hard, flat, levelled ground, containing no trees or bushes, and have a consistent texture and moisture content. This makes them perfect for speed fanatics. As such, land-speed record attempts have often been held at the Bonneville Salt Flats Racetrack. The salt flat stretches 75 km2 and is straight and long enough to give racers enough distance to accelerate and then slow down. Throughout the 20th century the fastest cars in the world have attempted to beat the world land-speed record here, with Gary Gabelich achieving 630 mph in 1970. More recently, the record attempts have moved to the Black Rock desert (another large flat, smooth barren area) and the current record (1997) stands at 763 mph, which is faster than the speed of sound!

The Blue Flame Rocket Car, driven by Gary Gabelich in 1970. It achieved speeds of over 600 mph on the Bonneville Salt Falts.
[Image: Troxx, Public domain, via Wikimedia Commons]
This is interesting, but what exactly is sodium chloride?Sodium chloride is an ionic compound, consisting of an ionic assembly of negatively charged anions and positively charged cations, resulting in a neutral compound with no net electrical charge. Electrostatic forces called ionic bonds hold the ions together. The ions in an ionic compound can be inorganic such as chloride (Cl-), or organic such as acetate (CH3COO-). Ions such as sodium (Na-+) and chloride (Cl-) are monatomic, or the ions can be polyatomic such as sulfate, (SO42-). This means sodium chloride is inorganic?Yes, sodium chloride is an inorganic chloride salt which has a positively charged sodium as a counterion. Its structure is a crystalline lattice structure with octahedral co-ordination. Each positively charged sodium atom has six neighbouring chlorine atoms of the opposite charge, positioned at the six vertices of a regular octahedron. It is thought of as a face-centred cubic (fcc) lattice, where two-unit cells can merge, forming a 3d checkerboard pattern. The larger chloride (Cl-) ions have an atomic radium of 181 pm and are arranged in a cubic array, however the smaller sodium (Na+) ions have an atomic radius of 102 pm and fill the cubic gaps. |
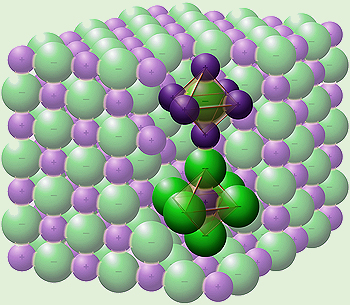 The sodium chloride octahedra in the crystal lattice. [Image: Goran tek-en, CC BY-SA 4.0 via Wikimedia Commons] |
Each sodium ion is electrostatically bonded to 6 chloride ions and vice versa, so sodium chloride is '6:6 co-ordinated'. With increased attraction between the negative and positive ions, more energy is released, which in turns causes the structure to be more energetically stable. Six chloride ions can co-ordinate to a single sodium ion before the chloride ions begin to touch, this would introduce repulsion into the crystal and decrease the stability. Therefore, sodium chloride is 6:6 co-ordinated as that is the maximum number of attractions to gain the maximum crystal stability.
Sodium ions (Na+) which are present in NaCl are essential for biological cells to work properly. As sodium chloride contains 40% sodium, it is an electrolyte with solutions conducting electrical currents. This is important for many bodily processes where electrical signals are required, notably in the brain, muscles, and the nervous system, and sodium ions can generate these electrical signals. When sodium quantities are too high or too low, it can cause cells to malfunction, and this can be dangerous or even fatal. Sodium is the major positive ion in bodily fluids and therefore the total concentrations of solutes (the osmolarity) is determined by the sodium concentration. For example, without enough NaCl, cells would lose water resulting in dehydration, low blood pressure and potentially death. Therefore, NaCl is needed to maintain the balance of fluid and minerals in our bodies
Salt is known as a solute (particles which are dissolved), and water is a solvent (a liquid dissolving the particles). When two fluids have a different concentration of solutes, water flows through a semi-permeable membrane from an area of low solute concentration to an area of higher solute concentration, causing the concentrations to equalise. This process is called osmosis. For bodily fluids, the fluid in cells is separated from the fluids in the blood by the cell wall (a semi-permebale membrane). Therefore, if the concentration of solutes in the blood does not equal that of a cell, the total volume of liquid inside a cell may increase or decrease. When cells contain too little salt, meaning the salt concentration is higher outside of the cell (a hypertonic environment), water will move across the membrane out of the cell causing the cell to shrink. Conversely, cells that contain too much salt, have higher concentrations compared to outside the cell (a hypotonic environment). Water will now move across the membrane into the cell taking in more water than the cell can sustain causing it to swell and potentially burst.

The effect of different salt concentrations on red blood cells.
Hypertonic = salt concentration outside the cell is higher than inside, so water leaves the cell by osmosis and the cell shrinks.
Isotonic = salt concentration outside the cell is the same as that inside, so the cell stays healthy.
Hypotonic = salt concentration outside the cell is lower than inside, so water enters the cell by osmosis and the cell bulges and bursts.
[Image: LadyofHats, Public domain, via Wikimedia Commons]
 A selection of isotonic sports drinks. These contain similar concentrations of salt and sugar as in the human body. |
How much salt should we be consuming, then?Salt is removed from the body through urine and sweat, and therefore needs to be constantly replaced. Athletes who lose a lot of salt via sweating often rehydrate with so-called isotonic sports drinks which contain similar concentrations of salt and sugar as in the human body, so keeping their salt levels constant. But ingesting too much salt also leads to health risks. Adults in the UK are recommended to have no more than 6 g of salt a day, which is equivalent to one level-teaspoon. This includes salt which is added during and after cooking and salt added to commercial foods. Disagreements about the amount of salt one should eat has arisen though, with the World Health Organisation (WHO) suggesting 5 g per day, 15% less than what is recommended in the UK. To help monitor daily salt intake, food packaging displays labels which shows the salt content of the product. |
 Why do foods have salt in them then?
Why do foods have salt in them then?Around 5,000 years ago, the Chinese realised that salt could be used to preserve food. Studies have found that salt is a good preservative as it reduces a food's water activity; the amount of water which is not bound to food molecules and can therefore support bacterial growth. The ability of salt to decrease the water activity can be explained by sodium and chloride ions interacting with the water molecules, meaning there is less unbound water available for bacteria to grow. With the invention of refrigerators and freezers, salt was no longer needed as a preservative, leading to a decline in the amount of salt consumed. An increase in salt has happened yet again in the food industry because it is added to food either by consumers or food producers. Salt helps improve the sensory properties of almost every food item, even making unappetizing foods ‘taste’ better. Think of bread, pasta, pizza, and processed meats, they all contain salt which could take us over our daily salt limit. For those familiar with high levels of salt in their food, its sudden removal can lead to foods tasting ‘bad’, with research considering an excessive intake of salt to be like 'an addiction'.
 Measuring blood pressure. [Image: rawpixel.com, CC0, via Wikimedia Commons] |
Is salt linked to heart disease?Yes, evidence from studies have proven a strong relationship between salt intake and cardiovascular disease. If too much salt is eaten, this results in a high concentration of salt in the bloodstream, which pulls water out of cells and into the blood. With more water present in blood vessels, a person’s blood pressure increases. High blood pressure results in the arteries being affected, as the force of blood pushing against artery walls is constantly too high. This results in the heart having to work harder to pump blood around the body. The increase in blood pressure is therefore a risk factor for heart attacks, heart failure and renal disease, and accounts for 62% of strokes, and 49% of coronary heart disease. A reduction in salt to the recommended level of less than 5-6 g/day therefore has major health benefits, especially for those who already suffer with heart failure. The progression of heart failure and associated symptoms are further exacerbated by a consistent high-salt diet as it aggravates water retention. As it stands, the consumption of too much salt means approximately 1.5 litres of fluid is being retained in the body, which will continue with high intakes of salt. So, it appears that high-salt diets are a killer as they can lead to heart disease, the most common cause of death around the world. Looking at the change in western diet, the number of food items with salt has greatly increased with the consumption of highly salted processed foods. While the UK national guidelines suggest a maximum of 6 g per day, 8.4 g are consumed daily by working-age adults, a daily excess of 40%. No wonder there is an estimated 1.89 million deaths each year associated with too much salt! |
 Packet of iodised salt. [Image: Ethantrott, CC BY-SA 4.0 via Wikimedia Commons] | So table salt is only sodium chloride?Actually, in most developed countries, table salt has small amounts (5-20 mg/kg) of iodine salts added to it as a public-health measure. Worldwide, iodine deficiency affects about two billion people and causes intellectual and developmental disabilities. It also causes thyroid-gland problems, including goitre. The good news is that these problems are entirely preventable by ingestion of iodine. Iodine deficiency can be addressed cheaply by deliberately adding small amounts of iodine to table salt. So-called 'iodised salt' is now widely available in most countries, and all table salt is iodised by law in many countries. An unusal exception to this is the UK, where iodised table salt is not the norm, although you can still buy it in certain supermarkets. Instead, milk is iodised, and this led to an 'accidental public health triumph' in that the high milk consumption in the UK virtually eliminated goitre from the population. However, with the increasing numbers of people choosing vegan foods (i.e. they don't consume diary products), a number of studies between 1995 and 2020 have begun to find iodine deficiency in British teenagers and pregnant women. It may be that vegans may need to take iodine supplements. Doesn’t salt also have a use in fire extinguishers?Yes, sodium chloride seems to be quite versatile as it is also used in specialist fire extinguishers to tackle Class-D flammable metal fires involving magnesium, sodium, and potassium. This type of fire extinguisher is powder based and can be recognised by being coloured red, with a dark blue panel above the instructions. When applied to a fire, the heat from the fire causes the NaCl to cake together forming a crust which stops air from having contact with the metals. |
 A Class-D fire extinguisher containing salt. [Image: By Firetech117 at English Wikipedia, CC BY-SA 3.0] |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25700058]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.25700058]