 The
majority of spiders possess neurotoxic venom.
These neurotoxins are multicomponent but contain three main groups of
toxic compounds:
The
majority of spiders possess neurotoxic venom.
These neurotoxins are multicomponent but contain three main groups of
toxic compounds: The
majority of spiders possess neurotoxic venom.
These neurotoxins are multicomponent but contain three main groups of
toxic compounds:
The
majority of spiders possess neurotoxic venom.
These neurotoxins are multicomponent but contain three main groups of
toxic compounds:
Low Mr polyamines (Mr less than 1000)
Polypeptides (Mr 3000-10,000)
High Mr proteins (Mr more than 10,000)
Other
venom components include inorganic ions and salts, free acids (eg. lactic acid),
glucose, nucleic acids, free amino acids and biogenic amines.
The exact role of these is unknown but they are thought to aid the
stability, delivery and effectiveness of the toxins.
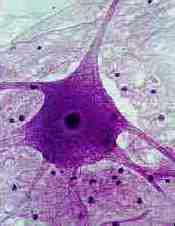
The excitability of the cell membrane and the transmission of electrical signals across a synapse are very important in the function nerve tissue. As a result, neuronal receptors, ion channels or membrane proteins involved in neurotransmitter release are attacked by most venoms.
Picture from reference 12
Polyamines
The structure of a spider polyamine consists of a hydrophobic, aromatic carboxylic acid region connected to a hydrophilic polyamine amide chain.

Polyamines work by blocking neuromuscular junctions in insects to prevent the release of the main neurotransmitter, glutamate, resulting in paralysis. These toxins tend to be specific for insects and not vertebrates.
Polypeptides
These attack ion channels and are the major components of spider toxins. Ion channels are proteins situated on the nerve cell membrane, through which ions can pass to move across the membrane. The channels control the electrical potential of the membrane and ionic balance so they are vital in neurotransmitter release. The different types of ion channel and examples of the toxins which affect them are discussed below.
Calcium channels are important in cardiac and muscular function. Voltage-dependent calcium channels are blocked by w-agatoxins (30-40 amino acid peptides) from Agelenopsis aperta which causes muscular paralysis due to prevention of neurotransmitter release. w-Agatoxins can be selective for calcium channels of different animal groups such as mammals, birds and insects.
Sodium channels of the voltage-dependent kind are present in nerve and muscle cells. They are targeted by m-agatoxins (36-37 amino acid peptides) which increase the amount of Na+ moving across the cell membrane to cause excessive presynaptic neural stimulation and massive neurotransmitter release. This causes hyperstimulation of post-synaptic receptors resulting in paralysis. These channels are attacked in the same way by d-atracotoxins from the Australian funnel-web spiders Atrax robustus and Hadronyche versutus which show significant toxicity towards humans.
Potassium channels control the duration and frequency of electrical signals so it is possible that they influence cardiac function. Voltage-dependent potassium channels are targeted by hanatoxins (35 amino acid peptides) from the Chile Rose tarantula (Grammastola spatulata). It is thought that they work together with sodium channel toxins to induce massive neurotransmitter release and paralysis.
Polypeptide toxins all have the same basic structure. A single polypeptide molecule is folded so that a b-sheet consisting of three strands is made. The overall structure of the peptide is termed a ‘cysteine knot’.
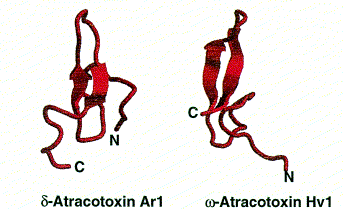
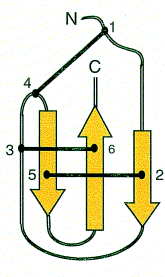
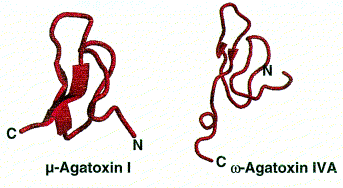
Images from reference 8 Structures of polypeptide toxins and schematic diagram of a cysteine knot
Proteins
An example of a neurotoxic protein is a-latrotoxin from the black widow spider. It is highly toxic to vertebrates and causes massive neurotransmitter release.
Enzyme proteins are used in necrotoxins. The active enzyme in brown recluse spider venom is sphingomyelinase D which causes the degradation of cell membranes and the development of painful lesions.