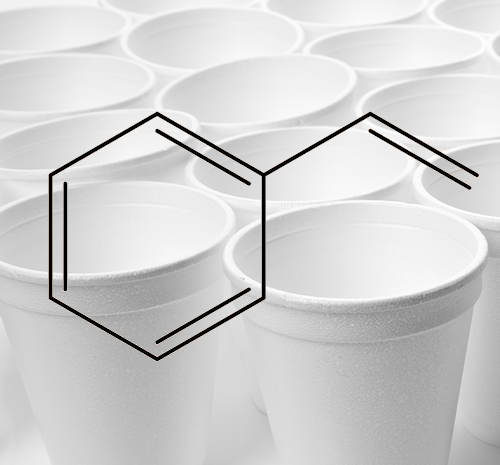
Styrene and polystyrene cups.
![]()
STYRENE
The building block for all those cups!
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month September 2021
Also available: JSMol version.
![]()
 Styrene and polystyrene cups. |
STYRENEThe building block for all those cups!
Simon Cotton
Molecule of the Month September 2021
|
Anything to do with Styria?No, the name is derived from styrax, a Latin word. How so?It goes back to the first time that styrene was isolated in 1839. A German named Eduard Simon got a volatile liquid from ‘storax’, the resin of the American sweetgum tree (Liquidambar styraciflua). This liquid he named ‘styrol’, publishing his findings in a paper entitled Ueber den flüssigen Storax (Styrax liquidus). We now call styrol ‘styrene’. Did he discover anything else?He found that if he left the ‘styrol’ around, it became more viscous. Thinking (not unreasonably) that the styrol had oxidised, he called the product styrol oxide. And was it?No, the process also takes place in the absence of oxygen, as two other contempory chemists, Blyth and Hofmann, soon noted. Within a further twenty years, the French chemist Berthelot worked out that what we would now call a ‘polymerisation’ happens. It was not until nearly a hundred years after Simon’s discovery that the nature of polystyrene, as it came to be called, was understood. |
 An American Sweetgum tree (Liquidambar styraciflua) in autumn. [Photo: Sanchezn, CC BY-SA 3.0 via Wikimedia Commons] |
No, it occurs in lots of natural sources, significant levels have been found in oats, coffee, tomatoes, fruits like peaches and strawberries and beef (for example) and particularly in cinnamon.
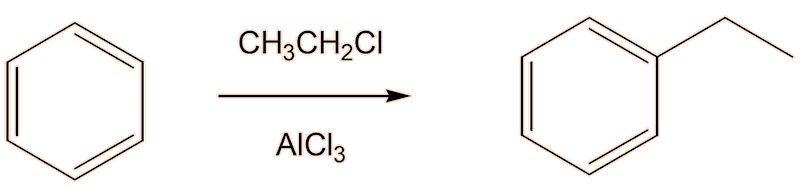
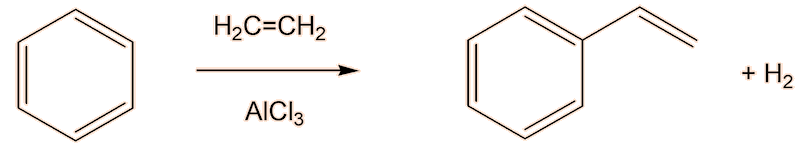
One way you could do it is first to make ethylbenzene, using a Friedel-Crafts alkylation of benzene by electrophilic substitution, using a catalyst like anhydrous AlCl3 or FeCl3.
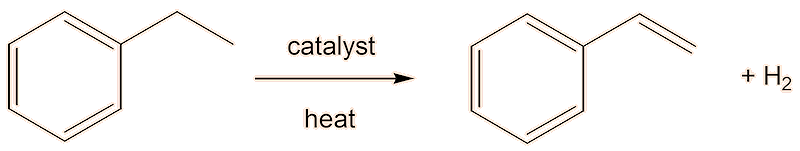
A second step dehydrogenates the ethylbenzene to styrene. Industrially, this is carried out in the gas phase in the presence of hot steam over a heterogenous iron(III) oxide catalyst.
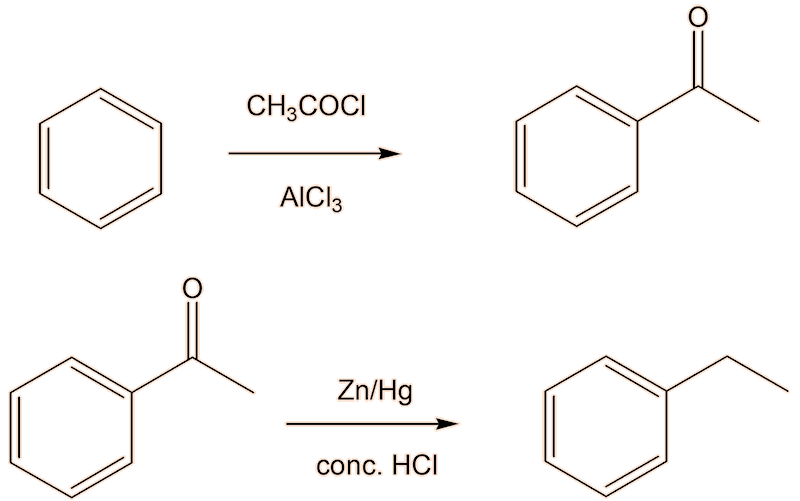
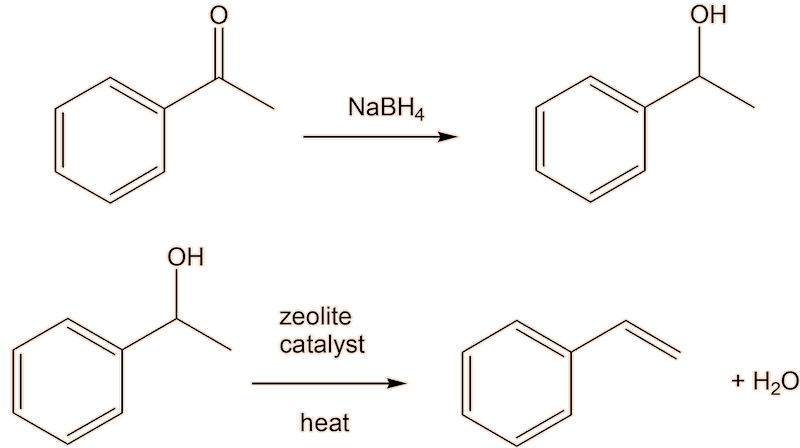
But there is a better way. The problem with alkylating benzene is that once you have introduced one alkyl group into a benzene ring, the alkybenzene is more reactive than benzene, so it is hard to stop the alkylation with ethylbenzene, so that the product is contaminated with diethylbenzene. Friedel-Crafts acylation forming the ketone phenylethanone (acetophenone), followed by reduction to get the ethylbenzene (here shown using the Clemmensen reduction) would be much better here.
A two-step conversion of phenylethanone to styrene via the intermediate alcohol 1-phenylethanol, which can be dehydrated to styrene, is an alternative.
Having said that, a lot of styrene is made industrially by reaction of benzene with ethene in the presence of AlCl3.
Well, you could do what Eduard Simon did back in 1839, but the chemical industry does things a bit faster by maing use of a catlyst.
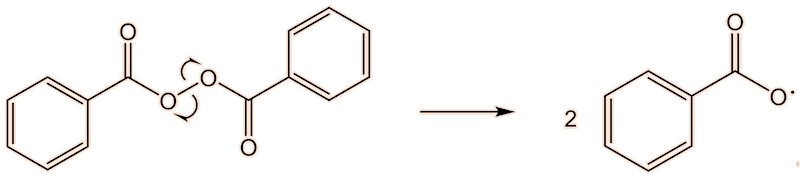
This requires a knowledge of how the polymerisation happens at the molecular level. A common catalyst used is benzoyl peroxide (see the MOTM for February 2017). This has a weak O-O bond, which undergoes homolytic fission, and readily splits into two benzoyl radicals. These, in turn, can lose CO2, forming phenyl radicals. This generation of radicals is termed an initiation step.
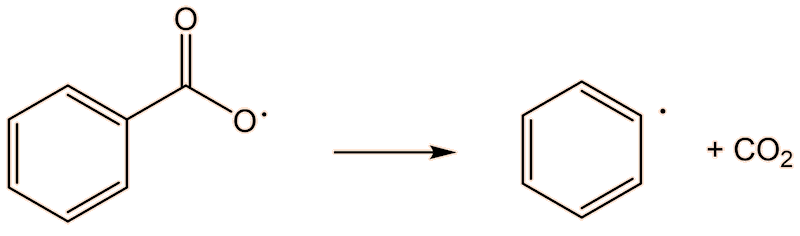
A phenyl radical can interact with a phenylethene monomer, in a propagation step; one radical disappears but is replaced by another one, so radicals are “propagated”.
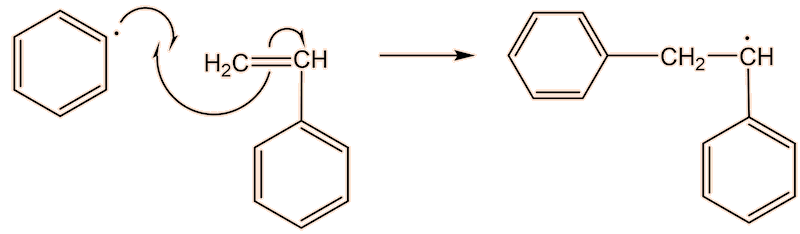
They are used to show the movement of electrons in a reaction mechanism when bonds are broken and formed. The arrows come from a source of electrons and end up where the electrons are going – usually forming a new bond.
No, it is because only one electron is involved, and is known as a “fishhook”. If two electrons were moving, it would have a full head.
No, the process repeats many times. Each step uses a radical and generates a new one.
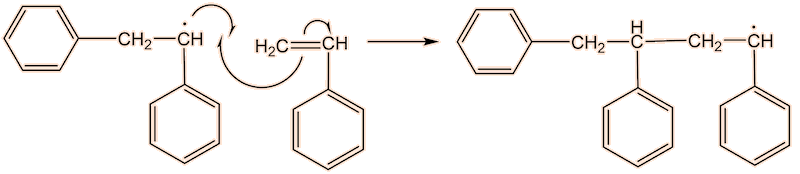
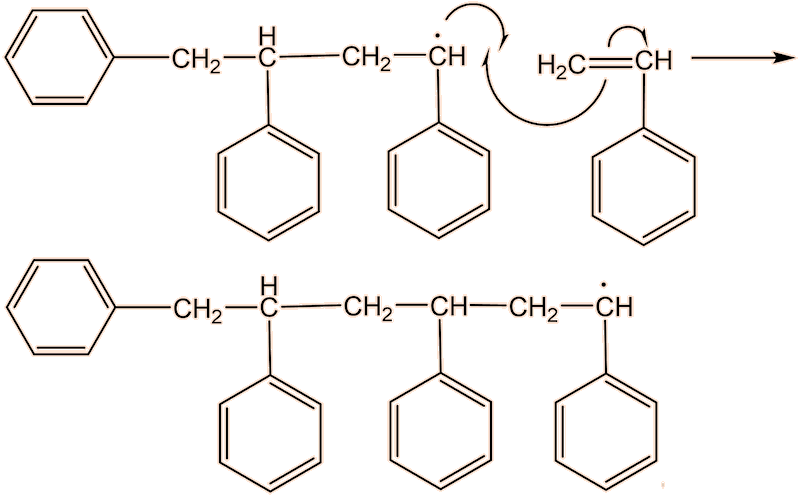
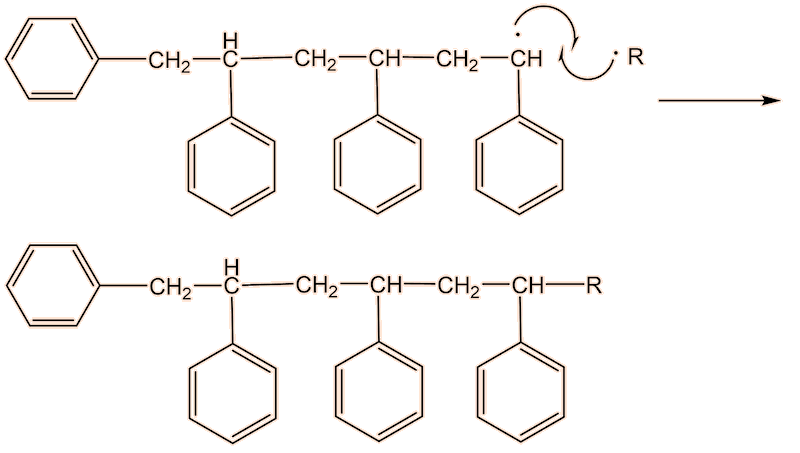
This process continues until either the monomer is all consumed, or else a termination step occurs – in practice, thousands of monomers are joined before this happens. The concentration of radicals is so low that collisions between a radical and a monomer molecule are much more frequent than collisions between two radicals. Termination steps use up all the radicals, so the reaction stops (hence the name termination); in the next equation R• refers to a non-specific radical.
The classical example of this polymerisation ‘in the home’ lies in wood fillers. These employ two tubes. One is a styrene-containing filler; the other, smaller tube, contains the benzoyl peroxide catalyst. The styrene is mixed with a thread of the catalyst (remember, it is a catalyst, so you do not need much of it whereupon the polymerisation begins. The mixture can be ‘worked’ and fitted into holes and crevices until it becomes too viscous. When solid, it can be smoothed out (and painted).
Polystyrene has many applications. There are many items found in our homes which are made out of hard polystyrene, such as plastic cutlery, DVD 'jewel' cases, the outside casing of computers, rulers, and toys, One application familiar to children of all ages is in plastic construction kits.

Most familiar though is in the form of ‘expanded’ polystyrene (EPS, a.k.a. ‘Styrofoam’). It was devised by Otis Ray McIntire, working for Dow Chemicals in 1941. Polystyrene beds are steam-heated to make them expand considerably; the expanded beads are packed into moulds of the shape that the manufacturers want to make (like a drink cup) and steam-heated again to fill the mould and all the beads are melted together. Expanded polystyrene is cheap to make, has a very low density (it is 95% air) and an excellent insulator, so it is well suited as a packaging material, as well as for keeping hot drinks hot and cold foods cold.

One issue with polystyrene, as usually made, is that it softens around 100°C, so that expanded polystyrene is vulnerable to softening. The reason for that lies in its structure. The phenyl groups can point either ’up’ or ‘down’. As normally produced, the polymer is made up of chains containing randomly oriented phenyl groups. This irregular structure means that the polymer chains cannot pack well in the solid state, reducing the intermolecular forces, hence its low softening temperature. This is known as the atactic form of the polymer.
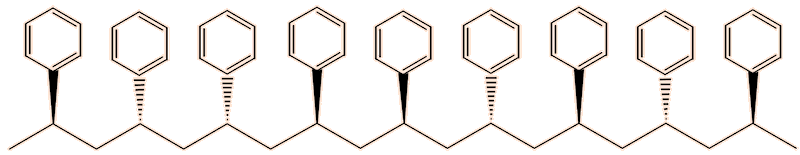
Atactic polystyrene
Another form is the isotactic form, in which all the phenyl groups point the same way, so that the chains pack better, leading to stronger intermolecular forces, leading to a melting point around 240°C.
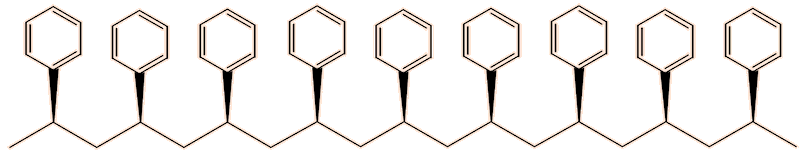
Isotactic polystyrene
In the syndiotactic form, the phenyl groups point alternatively ‘up’ and ‘down’. The intermolecular forces are even stronger, causing its melting point of 270°C.
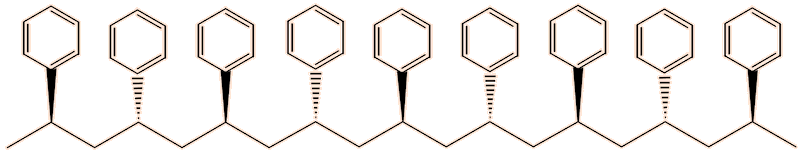
Syndiotactic polystyrene
As a consequence of the intermolecular forces, syndiotactic polystyrene crystallises well, unlike the isotactic form, though it is brittle. The synthesis of syndiotactic polystyrene uses a catalyst composed of organotitanium compounds such as [(C5H5)TiCl3] or [(C5H5)2TiCl2] together with methylaluminoxane (MAO).
The US Food and Drug Administration (FDA) are very careful about chemicals that are allowed to be used anywhere near foods, and for half a century it has said that polystyrene is quite safe to be in contact with foods and beverages. But styrene itself is a different matter. In the early hours of 7th May 2020, a styrene leak from a chemical plant at Visakhapatnam in the southern Indian state of Andhra Pradesh, killed 13 people and caused more than a thousand to fall sick enough to be hospitalised with breathing difficulties, sore eyes, headaches and skin rashes. It was reported that the styrene leaked from storage tanks that had been unattended for a month due to a coronavirus lockdown.
That's true. Expanded polystyrene (EPS) foam is a single-use material with no market for recycling. Thus, EPS products are quickly discarded and make their way onto beaches and into landfills. We’ve all seen discarded coffee cups or burger wrappers made of EPS, and these single-use items end up in the environment and make up 30% of the volume of all landfill space!
|
 Polystyrene forms 90% of the plastic pollution in the sea [Photo: EPE] |
Yes, the problem of keeping our streets clean is a minor one in comparison with keeping the oceans of the world plastic free. The lightweight composition and buoyancy of EPS allow it to easily float and travel far distances, and it all ends up in the ocean. Animals like seabirds can mistake large pieces of plastic floating on the surface for food, whilst if it sinks, a larger range of living things are vulnerable. Polystyrene foam can affect invertebrates in particular, reducing fertility, for example. Plastic pollution, and specifically EPS, comprise 90% of all marine debris, with single-use food and beverage containers being one of the most common items found in ocean and coastal surveys.
It can also create blockages. Tiny pieces, so called microplastics, are a particular cause for concern. They are not thought to affect human health significantly but the addition of microplastics to the oceans from diverse sources, estimated possibly as much as 100 million tons a year, is a threat to the life contained in the oceans.
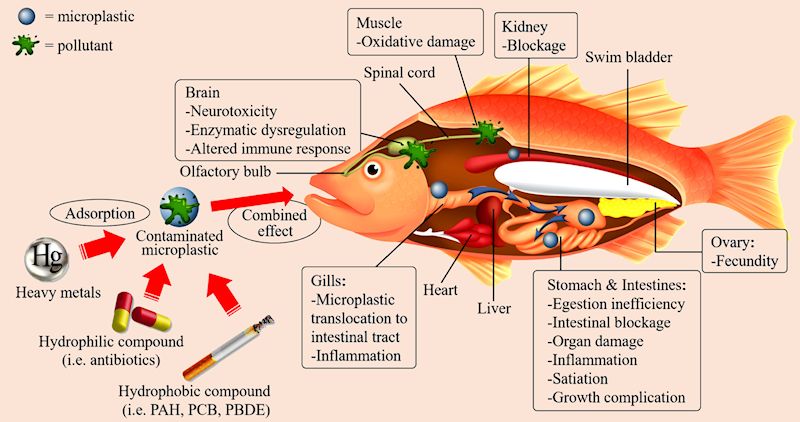
The impacts and combined effects of microplastic and pollutants toward marine organisms.
[Taken from: T.S.M. Amelia, et al., Prog. in Earth and Planetary Sci., 8, (2021) 12 via the Creative Commons 4.0 licence.]
Some States in the US have begun banning the use of EPS foam packaging. In 2019, Maine and Maryland both passed bills and will soon begin implementing fines for EPS packaging containers. New York City joined Los Angeles County, Seattle, Washington, DC, and San Francisco with a legislative ban on EPS foam as well. But in many other countries around the world, EPS still continues to be used, and discarded, in enormous quantities.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15044286]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15044286]