![]()
 |
Teflon
Layth Hendow (aged 15)
Molecule of the Month - June 2009 Also available: VRML, Chime Enhanced and JMol versions.
|
 |
 What is Teflon?
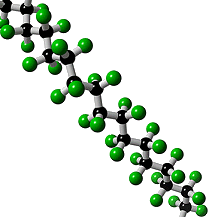
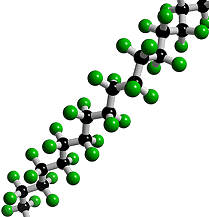
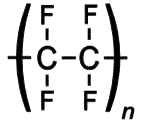
What is Teflon?Polytetrafluoroethylene, better known as Teflon, is a synthetic fluoropolymer. A synthetic fluoropolymer is a fluorocarbon-based polymer with multiple strong carbon-fluorine bonds, making it highly resistant to solvents, acids and bases.
 The discovery
The discoveryPolytetrafluroethylene was discovered by Roy Plunkett (photo, right), an American chemist from New Carlisle, Ohio in 1938. In an experiment to try and make a new CFC refrigerant, the tetrafluroethylene polymerized with the container it was in to make a white waxy substance called polytetrafluroethylene, later patented in 1941, and then commercially sold as Teflon in 1946.
Teflon is a polymer, which is made by joining together lots of smaller molecules called monomers. In this case, the monomer is tetrafluoroethene (TFE), and when polymerised it becomes poly-TFE, or PTFE as it's sometimes called.
Teflon's amazing properties are down to its structure. Like most polymers, Teflon has a carbon-based chain. However, instead of reactive C-H bonds which occur in most polymers, Teflon has all its hydrogens replaced by fluorines. These strong C-F bonds are extremely resistant to attack by any other reagents, making Teflon very inert. This means that no other molecules will react with or stick to Teflon. The exception is Teflon itself, which will stick to itself quite readily, forming thick layers or solid blocks. With a friction coefficient of <0.1, Teflon has the second lowest friction coefficient (surpassed only by diamond-like carbon), which makes it perfect for non-stick items e.g. pans. DuPont invented the non-stick pan coated with Teflon in 1956 and have manufactured it ever since. Teflon coatings are so slippery that they are the only material that a gecko cannot stick too. Also Teflon has a high melting point at 327°C so it won’t melt under the heat of cooking.




The trick is to use layers. First, the item to be coated (e.g. the pan) is sandblasted to create lots of little pits and scratches in the surface. Then a low viscosity primer liquid is poured in which flows into all the cracks, and heated to cure it into a thin solid layer. The identity of this primer is a commercial secret, but it is probably a polymer which contains a mixture of the C-F bonds of Teflon on one side with the C-H bonds of normal polymers on the other (e.g. a sort of semi-Teflon). The C-H side of the molecule happily sticks to the metal surface, leaving the C-F bonds pointing upwards. When the liquid Teflon is then poured on top of this, it will readily stick to the primer layer since it sees a C-F coated surface identical to itself. A further heating/curing stage allows the Teflon to bond to the primer and harden into a solid. Voila! A Teflon-coated pan.
|
|
|
Apart from cooking, Teflon has other uses in other industries. In machinery its slipperiness can be used where sliding is required, such as in coatings for gears and bearings. In the car industry, Teflon is the solution to the squeaky wiper blades that screech across your windscreen when it rains. The blade is coated with Teflon, which creates less friction on the windscreen and no noise! Teflon can protect fabric on furniture or carpets. Teflon repels liquids so that nasty spills on your carpet or sofa can be wiped up clean without leaving a stain. It is also used as an automotive lubricant, and even protects light bulbs from shattering. Because Teflon is a very good electrical insulator, Teflon tape can be used to cover exposed or broken wires. Teflon tape is also often used to wrap around the threads of bolts to allow a better seal.
Another non-stick polymer that is like Teflon, but is softer and more easily formed is FEP - fluorinated ethylene propylene. FEP was also invented by DuPont and is actually a copolymer of Teflon. It has similar properties to Teflon, however, it has a lower melting point 260°C so it can’t be used in cooking. However, because it is softer and is inert like Teflon, it can be used in electrical wiring as a putty to fill wires.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5426629]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5426629]