
The explosive that won WWI & WWII
![]()
Mike Thompson and George Innes
Rugby School, Rugby, UK
![]()
Molecule of the Month Dec 2014
Also available: JSMol version.
![]()

The explosive that won WWI & WWII
Mike Thompson and George Innes
Molecule of the Month Dec 2014
|
![Yellow TNT By Daniel Grohmann (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](yellowtnt.jpg) What is TNT?
What is TNT?The acronym TNT is short for 2,4,6 trinitrotoluene. It is an aromatic organic molecule with three nitro groups attached on the 2,4,6 positions of toluene (methyl benzene). TNT was first made in 1863 by Joseph Wilbrand, a German chemist, who was working on the production of dyes. Yellow TNT’s early use was not as an explosive, and this ‘hidden’ property was not discovered for another 20 years. From the mid 19th century until the end of WWII Germany was the world’s leading country in chemical research.
TNT is a yellow solid at room temperature (mp 80⁰C) and called Trolite in France and Trotyl in Germany. TNT has a detonation velocity of 6900 m/s, and in the early days of its use as an explosive it needed a primary explosive such as fulminate to detonate it.
TNT is made from toluene using a nitrating mixture (conc. sulfuric & conc. nitric acid). The methyl group has a positive inductive effect (electron releasing) making the nitration of toluene faster than benzene. The methyl group is also ortho-para directing stabilising the carbocation intermediates in the 2 and 4 positions on the arene ring.
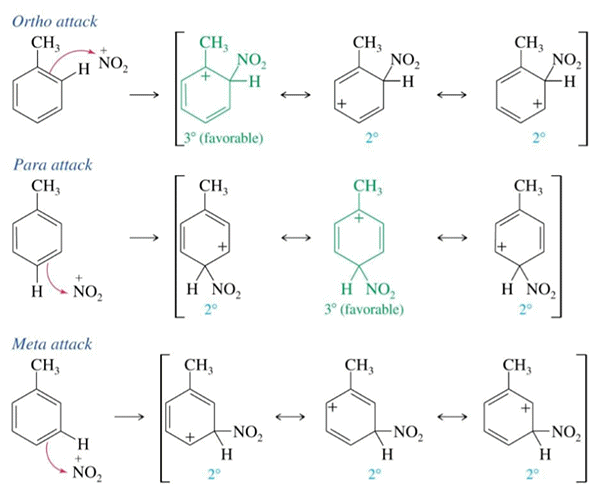
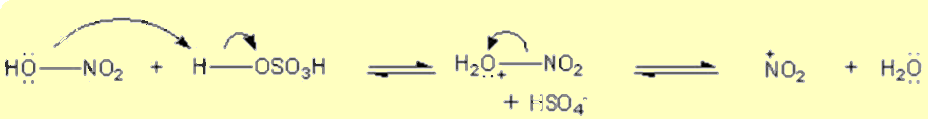
Generation of the electrophile, nitryl cation (NO2+) is from the reaction between H2SO4 and HNO3.
Nitration goes via mononitrotoluene (MNT), dintrotoluene (DNT) and then trinitrotoluene (TNT). A laboratory method is not to be recommended as a license is needed to make explosives which are highly regulated and require specialist training and equipment. The dangers of TNT are well documented.
During WWI the 8th Viscount Chetwynd was commissioned by the government to set up the National Shell filling factory at Chilwell in Nottinghamshire. During the course of the ‘Great War’ they produced 19 million shells at this site. Due to the great expense of TNT it was mixed with ammonium nitrate to make an explosive called Amatol which helped allow the TNT, which was in short supply, go further. Ammonium nitrate is an oxygen-rich molecule so provides extra oxygen to TNT during combustion ensuring a complete and more exothermic combustion. TNT is not shock sensitive which gave it an advantage over other explosives being used at the time like picric acid. However, the inevitable happened on the 1st July 1918, when the factory exploded injuring 250 and killing 134 people. The blast was so loud it was hear over 20 miles away from the site. A memorial to these brave factory workers is found in St Mary’s Church, Attenborough.
![Canary girls By British official photographer : Nicholls, Horace [Public domain], via Wikimedia Commons](canary.jpg)
Women workers with shells in Chilwell filling factory 1917
One side-effect of working with TNT was that the skin of the workers (mainly women) turned yellow. These women were known as the ‘Canary girls’. It is believed that over 400 women died from exposure to TNT during the course of WWI.
TNT’s superiority to other explosives was demonstrated at the naval battle of Jutland (1916) where the German TNT filled shells penetrated the British ships before exploding. This led to more damage than the British shells which detonated on initial impact. Other advantages of TNT is that its relatively low melting point (80oC) allowed it to be poured into shells more easily.
In 1941 a brilliant British aircraft designer, Sir Barnes Wallis, was working on an experimental bomb to destroy the dams in the heart of the industrial heartland of the then third Reich. The bouncing bomb contained the ubiquitous TNT. In the final attack the bomber had to fly at 220 mph at a height of 60 feet and release the bomb exactly 425 yards from the Mohne dam’s wall. Channel 4 (UK television station)produced a television programme Dambusters: Building the Bouncing Bomb which was first shown on 2nd May 2011.
![Bouncing bomb animation From Dake [GFDL (http://www.gnu.org/copyleft/fdl.html), CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/) or CC-BY-SA-2.5-2.0-1.0 (http://creativecommons.org/licenses/by-sa/2.5-2.0-1.0)], via Wikimedia Commons](Bouncing_bomb_dam.gif)
The movie Iron Man 2 features many tracks by the heavy-rock band AC/DC. One of their iconic songs is TNT which contains these lyrics:
'Cause I'm T.N.T., I'm dynamite |
 |
Whilst AC/DC are undoubtedly one of the world’s greatest rock bands their chemistry leads something to be desired as dynamite is based on the explosive nitroglycerine. Perhaps the band were using the word 'dynamite' as a slang term for something very dangerous? This link shows the power of TNT an explosive which the younger generation will have heard of through the computer game Minecraft.
The equation for the combustion of TNT is:
2CH3C6H2(NO2)3 (s) 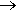 3N2 (g) + 5H2O (g) + 7CO (g) + 7C (s)
3N2 (g) + 5H2O (g) + 7CO (g) + 7C (s)
For every 2 moles of TNT we can see that 15 moles of gas is produced giving a large increase in volume of gas, which usually amounts to 1 gram of TNT producing 1 litre of gas. From the stoichiometric ratio in the balanced equation there is a very high increase in entropy change. On explosion, TNT creates a high velocity shockwave because a large volume of gas is produced in a very short space of time.
We can also see that TNT is a self-oxidising molecule as there is enough oxygen in the molecule for it to combust without the need for extra oxygen from other sources. Although not all the carbon has been oxidised as there is not enough oxygen in TNT for total oxidation to occur. To get around this problem and maximise the power of a TNT explosion it can be mixed with an oxygen-rich explosive to ensure total combustion of the carbon.
Amatol is a 1:1 to 1:4 mixture of TNT and ammonium nitrate used to demolish buildings. Torpex a mixture of TNT & RDX (cyclonite) and aluminium used to create large underwater shockwaves when torpedoes hit their target.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5398405]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5398405]