|
TramadolThe painkiller used by racing cyclists
|
 |
|
TramadolThe painkiller used by racing cyclists
|
 |
No, it’s a strong painkiller.
In his new autobiography, the former professional cyclist Michael Barry has claimed that before his retirement in 2012 he used tramadol – which is a legal medication – while cycling in the Sky team. It could help a cyclist cope with a “pain barrier” and so improve performance slightly, maybe enough to create an “edge”. Some people have referred to its use in “finish bottles” containing a mixture of a painkiller like tramadol and a stimulant like caffeine. Thomas said:
“When I crashed and broke ribs on the second day of the Tour de France, I took tramadol to alleviate the pain. The drug made me feel slightly euphoric. It made my legs feel painless. I could push harder than normal. It was as performance enhancing as any banned drug I had taken, but with a major difference: it was legal.”
 |
 |
Michael Barry |
Chris Froome |
Tramadol reportedly can have side effects, including dizziness and drowsiness, which could endanger other nearby riders, not just the person taking it. Team Sky has said that its use is no longer allowed, and have called for it to be outlawed by its regulation by WADA, the World Anti-Doping Agency.
Tramadol is not banned by WADA, but has been on their “Monitored List” since 2012. This means that its use is tested for in competition, “in order to detect patterns of misuse in sport.”
No one knows. In his autobiography, the British cyclist Chris Froome (winner of the 2013 Tour de France) says that “I do not take any painkillers or stimulants while training or racing”. He says “I do have a finish bottle on occasion, but it contains nothing more than a double espresso” (i.e. caffeine).
It’s been around for over 30 years, being developed (from 1962) by the German company Grünenthal, and is marketed through much of the world under various trade names, including Acugersic (Malaysia), Mabron (some Eastern European countries as well as parts of the Middle and Far East), Ultram (USA), Zaldiar (France and much of Europe, as well as Russia) and Zydol (UK and Ireland).
Tramadol is used to treat moderate to severe pain, much stronger than a headache. Save your paracetamol for that.
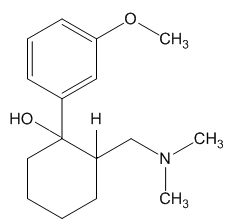
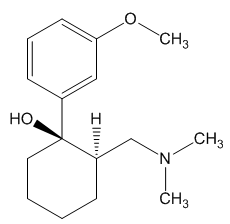
Tramadol is marketed as a racemic mixture of both R and S stereoisomers. It is a μ-opioid receptor agonist, like morphine, but much less active. It inhibits reuptake of the neurotransmitters serotonin and norepinephrine, suggesting that it lifts mood and thereby may dull the brain's perception of pain.
 |
 |
|
|
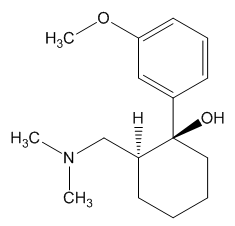
In the body, tramadol undergoes demethylation to several metabolites by a Cytochrome P450 enzyme (CYP2D6) in the liver, the most important of these products being O-desmethyltramadol. O-desmethyltramadol has a much stronger (200x) affinity for the μ-opioid receptor than tramadol, so in effect tramadol is a prodrug.
 |
 |
|
|
Not everyone’s liver works identically. Around 6% of the Caucasian population has a reduced CYP2D6 activity (hence reducing metabolism), so there is a reduced analgesic effect with Tramadol. These people require a dose increase of 30% to get the same pain relief as the norm. A case has been reported of a patient where, following an overdose, their ultrarapid tramadol metabolism led to excessive norepinephrine levels, with near-fatal consequences.
There is concern that it is being abused more generally. Annual deaths from tramadol in the UK have risen from 1 in 1996 to a record of 175 in 2012 (doubling over the preceding four years). The (UK) Advisory Council on the Misuse of Drugs (ACMD) recommended closer control of O-desmethyltramadol in 2012. It isn’t just in the UK that there are worries. Tramadol abuse is a particular concern in Egypt and Gaza, with some 30% of males aged 14-30 using it in Gaza, some being severely addicted. Smuggling of it is said to be rife. Because tramadol inhibits reuptake of the neurotransmitters serotonin and norepinephrine, there are reports of seizures when taken at the same time as antidepressants like Fluoxetine (Prozac), though the main problem is with Monoamine oxidase inhibitors (MAOIs). And the side-effects of Tramadol vary from person to person; in one slightly bizarre case, when a 74-year-old man was treated with tramadol, he soon started to experience auditory hallucinations; “two voices singing, accompanied by an accordion and a banjo, singing songs, songs by Josef Locke - old songs”.
In the area of “legal highs”, a disturbing development is a drug blend known as “Krypton”. This isn’t the noble gas, but a mixture of O-desmethyltramadol with Kratom (Mitragyna speciosa, a medicinal plant that originates in SE Asia, seemingly the local equivalent of khat), which contains an alkaloid mitragynine which is also a μ-receptor agonist. Several fatalities have been linked with its use, notably in Sweden.
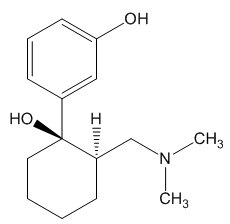
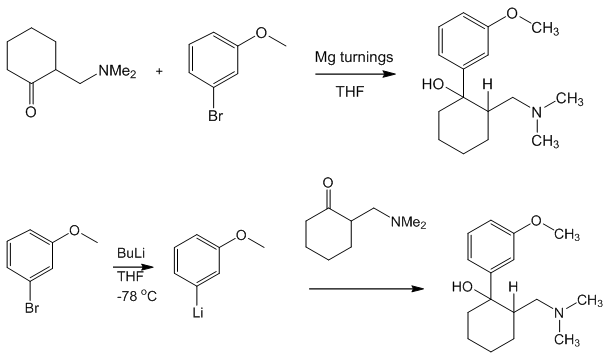
Yes and no. It was synthesised by chemists at the German company Grünenthal and brought to the market in 1977. It can readily be made by nucleophilic attack of a Grignard or RLi species upon a carbonyl group.
In 2013 it was reported that tramadol had been discovered at relatively high concentrations in the roots of the African peach or pin cushion tree (Nauclea latifolia), which has a long tradition as a folk remedy. It was suggested that the plant was indeed synthesising Tramadol, which would have been a rare case of a medicinal molecule being synthesised in the lab before it was discovered in Nature.
Subsequently, however, Tramadol was found in trace amounts in soil and water samples in northern Cameroon; it was pointed out that Tramadol is widely available in markets in that country and was widely used in both humans and cows. 14C mass spectroscopy on Tramadol both from contaminated soil samples and from pills bought on a market showed the absence of radiocarbon (14C), demonstrating that it had been made from 14C-free precursors, excluding a biochemical plant-based synthesis.

The African pin-cushion tree (Nauclea latifolia)
![]()
![]()