![]()
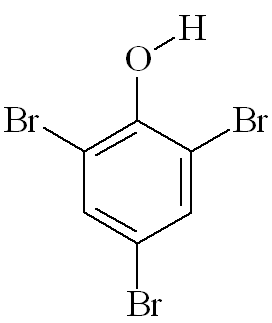
2,4,6-Tribromophenol (TBP)
(and some other natural organobromine compounds, notably Tyrian Purple)
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month December 2011
Also available: JSMol version.
![]()
|
2,4,6-Tribromophenol (TBP)(and some other natural organobromine compounds, notably Tyrian Purple)
Simon Cotton
Molecule of the Month December 2011
|
 |
 I thought that 2,4,6-tribromophenol was only made in the lab while testing for phenol?
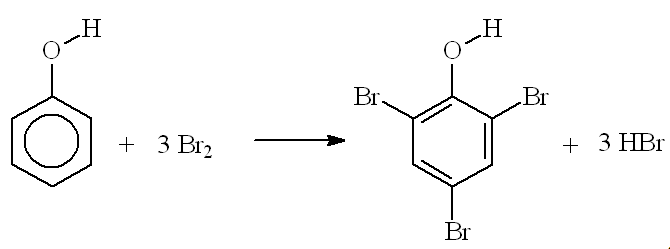
I thought that 2,4,6-tribromophenol was only made in the lab while testing for phenol?You are right that phenol reacts immediately with bromine water at room temperature forming a white precipitate of 2,4,6-tribromophenol (see photo, right); this has an antiseptic smell, like the TCP you buy in chemists' shops.
The OH group "activates" the benzene ring by increasing the electron density - especially at carbons 2, 4 and 6. This makes it more reactive (attractive) to electrophiles, so that three hydrogen atoms are substituted by bromines, not just the one replaced when you brominate benzene - this is much more difficult, and requires a catalyst.
Several, particularly as a fungicide and wood preservative and for making flame retardants, with some 10,000 tonnes produced a year.
There are worries. Scientists have found that 2,4-dibromophenol and 2,4,6-tribromophenol both disturb cellular Ca2+ signalling in neuroendocrine cells; bromophenols have been viewed as potential endocrine disruptors. "Environmental levels" of TBP have been reported to affect reproduction in zebrafish.
Not at all, 2,4,6-tribromophenol and other bromophenols are natural metabolites of various marine species, notably brown algae; 2,4,6-tribromophenol is found in a wide range of organisms, including seaweed and sponges. It appears that bromophenols are major contributors to the "ocean-like" flavour of seafood as fish have food sources that include bromophenol-rich algae. For example, they are believed to contribute to the taste of shrimp from the Gulf of Texas, and bromophenols have been added to the feed of farmed shrimp, though it seems that they cannot add that special je-ne-sais-quoi.




It has been suggested that bromophenols may be defence molecules against predators, but an examination of marine worms revealed little evidence for this. Research has shown that bromophenols may have several biological roles and that they could have some medicinal applications (antioxidant, antimicrobial, anticancer, anti-diabetic, and anti-thrombotic effects have been mentioned).
It could be worse. TBP is found in lots of consumer products at very low levels, and microbial metabolism in products treated with TBP is known to produce 2,4,6-tribromoanisole (TBA), which has a distinct musty smell and can lead to product recalls, as it has a very low detection threshold (reported as 0.08-0.3 parts per trillion in water and 2-6 ppt in wine). TBA leaches from wood used to make wine casks and can be responsible for "cork taint" in wines, giving them an unpleasant smell and potentially making them undrinkable.
The chlorinated analogues of this, 2,4,6-trichloroanisole (TCA) and 2,3,4,6-tetrachloroanisole (TeCA) have also been implicated in "cork taint".
 Are there any other important natural organobromine compounds?
Are there any other important natural organobromine compounds?Hundreds, notably 6,6'-dibromoindigotin, the major component of the dye known as Tyrian purple, a bromine containing analogue of Indigo, the most famous dye of antiquity.
It was the colour used for the togas of Roman emperors; 1500 years ago it was a capital offence for anyone other than an Emperor to wear it. The image above right is of a 6th Century mosaic from Ravenna, Italy, depicting the Byzantine Emperor Justinian I clad in a Tyrian purple robe. Once upon a time Catholic cardinals wore purple, but nowadays the colour is scarlet. The process for making Tyrian purple fell into obscurity after the fall of Constantinople (1453) and it is only recently that scientists have rediscovered how it was made.
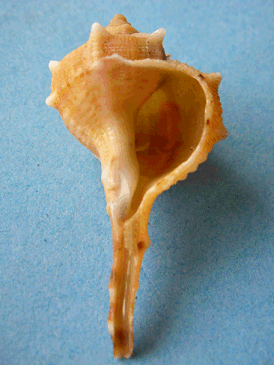 Where did it come from?
Where did it come from?Sea snails, notably on the Mediterranean and the Atlantic coasts, particularly Bolinus brandaris (photo, right), Murex brandaris and others including Murex trunculus) secrete very small amounts of a yellow liquid; on exposure to sunlight and the air, it turns to Tyrian Purple. It got its name from Tyre in Phoenecia. Paul Friedlander, the first man to find out the structure of Tyrian Purple (1909) needed 12,000 Murex brandaris snails to obtain 1.4 grams of Tyrian purple.
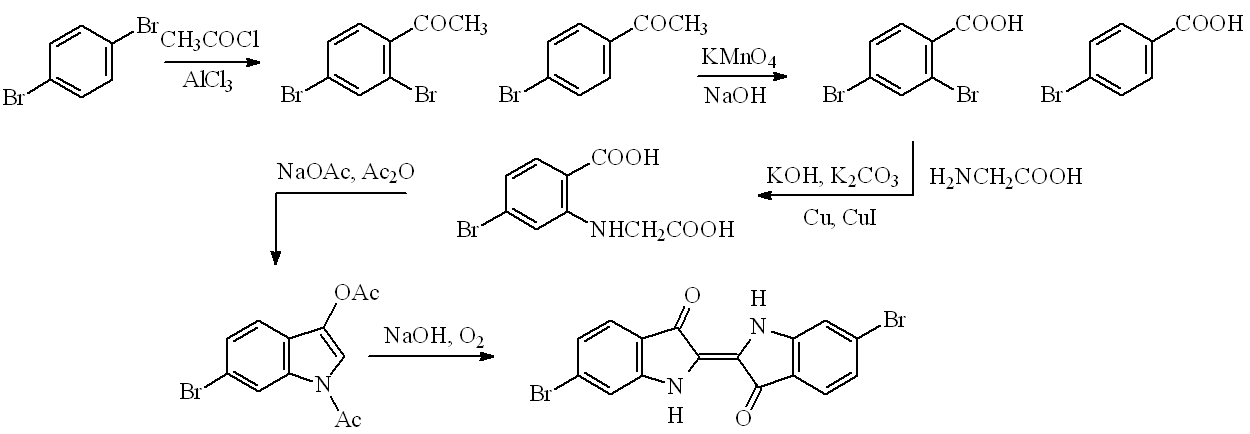
Chemists have long tried to find better syntheses of Tyrian purple. Two new routes have been recently described; one starts from 4-bromo-2-nitrobenzaldehyde, but better is a new five-step, high-yield (25%) route starting from 1,4-dibromobenzene. This undergoes acetylation principally to 2,4-dibromoacetophenone, which can be oxidised (basic permanganate) to 2,4-dibromobenzoic acid. This undergoes Ullmann condensation with glycine forming a substituted phenylglycine, which can be converted to 6-bromodiacetylindoxyl by a Claisen condensation. The latter on alkaline hydrolysis in the presence of oxygen affords 6,6-dibromindigotin, as a result of a dimerisation.
![]()
Chapman and Hall Combined Chemical Dictionary compound code numbers: BJD51-Y (2,4,6-tribromophenol); BJD55-C (2,4,6-tribromoanisole); HHT48-E (dibromoindigo); (props, bibliography)
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255614]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255614]