![]()
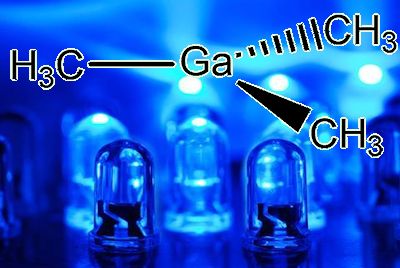
Trimethylgallium
The precursor to gallium nitride and blue LEDs
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month February 2023
Also available: HTML version.
![]()
|
TrimethylgalliumThe precursor to gallium nitride and blue LEDs
Simon Cotton
Molecule of the Month February 2023
|
 |
Ga(CH3)3, or, if that is too long for you to write, Me3Ga.
What’s it like?At room temperature, it is a colourless liquid, which freezes at -16°C and boils at 56°C. And it’s pyrophoric. What does that mean?Expose it to air, and it bursts into flame. That must limit its uses.Not so, as we will see. |
 GaMe3 is a colourless liquid. It is usually sold in sealed metal canisters for use in the semiconductor industry. |
Keep it away from air or moisture – then it is stable to over 400°C. A study of its thermal decomposition indicates that its decomposition starts at ~480°C, forming largely methane, with a little ethane, plus elemental gallium. The mass spectrum under these conditions shows the presence of Ga+, Ga(CH3)+, Ga(CH3)2+ and Ga(CH3)3+ ions.
There are several ways. One is by the reaction of gallium metal with dimethyl mercury. The latter substance has the serious disadvantage of being deadly poisonous (MOTM October 2003).
2 Ga + 2 Hg(CH3)2 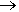 2 Ga(CH3)3 + 3 Hg
2 Ga(CH3)3 + 3 Hg
So you are better off choosing the reaction of gallium chloride with trimethylaluminium. Although trimethylaluminium is also pyrophoric, at least it is not poisonous.
GaCl3 + Al(CH3)3 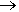 Ga(CH3)3 + AlCl3
Ga(CH3)3 + AlCl3
There are other ways, for example:
2 GaCl3 + 3 Zn(CH3)2 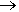 2 Ga(CH3)3 + 3 ZnCl2
2 Ga(CH3)3 + 3 ZnCl2
You can use a Grignard reagent, a solution of CH3MgBr in diethyl ether.
GaCl3 + 3 CH3MgBr  Ga(CH3)3 + 3 MgBrCl
Ga(CH3)3 + 3 MgBrCl
The disadvantage of this method is that the solvent ether, being a Lewis base, coordinates to the gallium, so the reaction product is Ga(CH3)3.OEt2.
In the gaseous state, it exists as isolated Ga(CH3)3 molecules, with a trigonal planar coordination sphere of gallium.
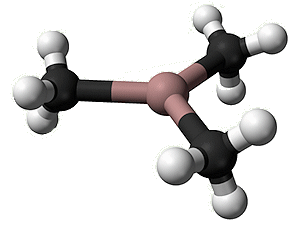 Trimethylgallium |
Gallium is involved in three covalent (two-electron) bonds. Mutual repulsion means that these electron pairs keep as far away from each other as possible, so that the C-Ga-C bond angles are 120°, as in BF3. VSEPR rules, OK.
In the solid state the molecules weakly associate by long gallium-to-methyl interactions; in one type of crystal, the Ga(CH3)3 molecules associate weakly into tetramers, whilst in the other crystal form, the molecules stack in a ladder-like structure.
At normal temperatures, it reacts with Lewis bases like ammonia, amines and tertiary phosphines, as it does with diethyl ether. For example:
Ga(CH3)3 + NH3  Ga(CH3)3.NH3
Ga(CH3)3.NH3
Ga(CH3)3 + NMe3  Ga(CH3)3.NMe3
Ga(CH3)3.NMe3
Because the gallium atom is now surrounded by four electron pairs, the repulsion between them is minimised when they are arranged tetrahedrally (just as in CH4, methane, MOTM December 2007).
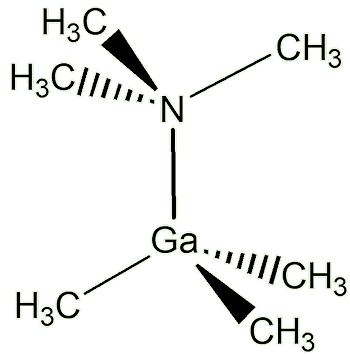 Ga(CH3)3N(CH3)3 |
Similar complexes have been made with lots of other ligands, such as Me3NO, Me3PO, Me2SO, NHR2 (R e.g. Me, Et, cyclohexyl). With bidentate ligands, 2:1 complexes are usually obtained, with two moles of Me3M per mole of Iigand, like [Me3Ga.Me2N(CH2)3NMe2.GaMe3] and [Me3Ga.PPh2CH2CH2Ph2P.GaMe3].
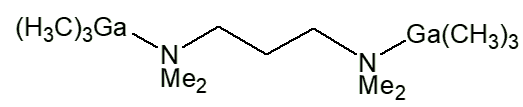 Me3Ga.Me2N(CH2)3NMe2.GaMe3 |
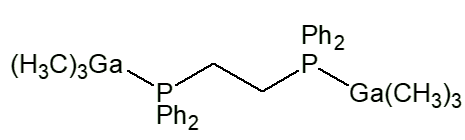 Me3Ga.PPh2CH2CH2Ph2P.GaMe3 |
There is also a remarkable compound with potassium fluoride.
It was made as a way of purifying trimethylgallium, as a way of eliminating oxygen as an impurity, which adversely effects the properties of semiconductors.
On refluxing a mixture of KF with Ga(CH3)3 in xylene, colourless crystals analysing as Ga(CH3)3.KF form on cooling. These actually have the structure (KF)4(Ga(CH3)3)4, which are based on a slightly distorted cubic K4F4 core, which resembles a fragment of a lattice of crystalline potassium fluoride. Each fluorine is attached to a Ga(CH3)3.
These crystals decompose on heating above 250°C, affording pure trimethylgallium.
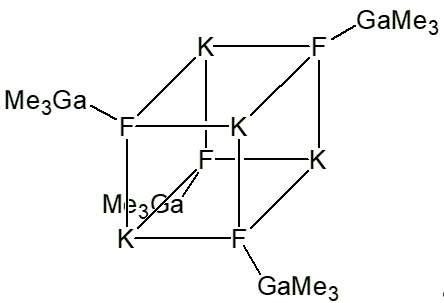 Ga(CH3)3.KF |
As we have seen, at room temperature, trimethylgallium reacts with ammonia forming the simple adduct Ga(CH3)3.NH3. At slightly higher temperatures (above 100°C), a different reaction occurs, forming a cyclic compound (CH3)2Ga(NH2), with the elimination of methane. In the solid state, this is a trimer, with a cyclic structure.
n Ga(CH3)3 + n NH3 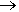 [(CH3)3Ga(NH2)]n + nCH4
[(CH3)3Ga(NH2)]n + nCH4
 |
|
| (CH3)2Ga(NH2) |
At even higher temperatures, further decomposition takes place, forming GaN.
overall: Ga(CH3)3 + NH3  GaN + 3 CH4
GaN + 3 CH4
Similarly, hydrazine, which forms an adduct at low temperatures, reacts with Ga(CH3)3 at higher temperatures to form GaN.
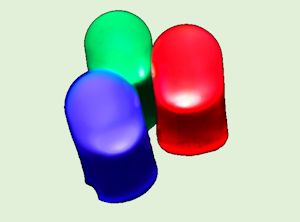 So?
So?Gallium nitride is a semiconductor with a direct band gap. That makes it useful for blue- and UV light-emitting diodes (LEDs) and also laser diodes.
Blue LEDs were the reason for the award of the 2014 Nobel Prize for Physics, to Isamu Akasaki, Shuji Nakamura and Hiroshi Amano.
What's the big deal about blue LEDs?Red, yellow, green and orange LEDs (light-emitting diodes) have been around since the 1970s and are part of most electronic devices. But blue LEDs were always difficult to make. Once blue LEDS made from GaN were invented, this meant the 3 primary colours that make up white light were now possible. This enabled RGB (red, green, blue) displays to be manufactured, and paved the way for LED televisions, computer monitors and even traffic lights. LEDs use far less power than standard lightbulbs for the same brightness, so switching all the traffic lights in a city to LEDs can save tens of thousands of pounds in electricity bills a year. Also, mixing all three colours together gives white light, allowing household lightbulbs also to be replaced by energy-saving LEDs. And from a chemistry point of view, UV LEDs can be used to drive chemical reactions instead of heat. This not only saves money, but gives the opportunity to instigate only the specific reaction you want, eliminating unwanted side reactions, as well as allowing stero-specific reactions to be developed. |
 |
Trimethylgallium can also be used to make Ga2O3 thin films using the reaction between trimethylgallium and oxygen plasma. The Ga2O3 has several applications including field-effect transistors.
So trimethylgallium is used on quite a large scale for a ‘speciality’ chemical.
I’d rather think of trimethylgallium as MeMeMeGa.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.20384232]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.20384232]