![]()
Vitamin B2 (Riboflavin)
More than just a food colouring
![]()
![]()
Molecule of the Month February 2018
Also available: JSMol version.
![]()

A bright-yellow solution of riboflavin
Vitamin B2 (Riboflavin)More than just a food colouring
Molecule of the Month February 2018
|
 A bright-yellow solution of riboflavin |
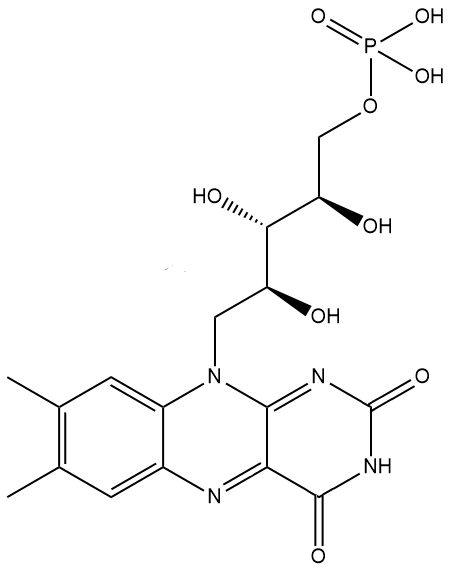
Yes, in that instance it’s being used as a colouring additive due to its bright yellow-orange colour, and has been given the European E-number E101. The phosphate version, which is more soluble in water, is also used as a yellow food colouring (E101a) and is actually the principal form in which riboflavin is found in cells and tissues.
 |
 |
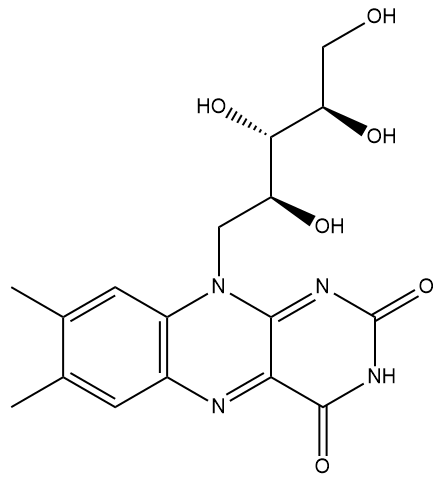
| Vitamin B2 (Riboflavin) |
riboflavin-5′-phosphate (a.k.a. flavin mononucleotide, FMN) |
What’s so special about it?Well, it’s one of the water soluble ‘B’ vitamins that are essential in various cell metabolic processes, such as those that convert sugars into energy. It is crucial in the metabolism of fats, ketone bodies, carbohydrates, and proteins, and supports the immune system and nervous system. Riboflavin is designated vitamin B2, while others include thiamine (B1), niacin (B3), etc. Humans, and other animals, cannot synthesise riboflavin, so they have to ingest it in the food they eat. What sort of foods?It’s found in eggs, many types of green vegetables, milk, cheese, mushrooms, almonds, and meat. And it’s often added to baby foods, breakfast cereals, pastas, flour and bread as a food supplement. It is heat stable, so cooking doesn’t easily destroy it, but it is readily destroyed by ultraviolet light or sunlight. That’s why the labels of many foods suggest they’re stored in a place away from direct sunlight. |
 Foods rich in vitamin B2. |
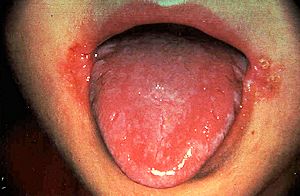 One of the symptoms of vitamin B2 deficiency is a red swollen tongue and inflammation of the lips. [Image: http://medlibes.com/entry/vitamin-b2-deficiency] |
Why add it to foods –what happens if you don’t get enough of it?Riboflavin deficiency causes some health issues, such as cracking of the skin at the corners of the mouth and inflammation of the lips, swollen red beefy tongue, sensitivity of eyes to light, and itching of the skin on the face. And in animals it can stunt growth. Sounds bad…Yes, but it’s nowhere near as severe as the diseases caused by deficiencies in other vitamins, such as B1 (beri beri), C (scurvy), or D (rickets). In most developed countries nowadays vitamin B2 deficiency is rare, due to good diet and those food additives. But surprisingly, in the developing world, and in war zones and famine areas, riboflavin deficiency is one of the most common nutritional deficiencies. However, because it is not linked to any serious clinical conditions, little attention is paid to it. Indeed, there have been no reports that riboflavin deficiency has ever proven fatal in humans. |
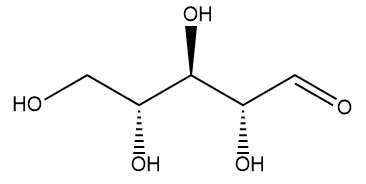
Well, the name comes from its two component molecules, a sugar called 'ribose' and 'flavin' (from Latin flavus, meaning 'yellow') which is the name given to a group of yellow compounds based on isoalloxazine. The flavin part of the molecule is the important bit, as it can accept either one electron in a two-step process or two electrons simultaneously. It can also be reduced by the addition of a hydrogen atom onto the N on the central ring. This flexibility in oxidation-reduction reactions makes the flavin group a very important biological species in a host of biochemical pathways. The ribose group simply serves to make the molecule more water soluble.
 |
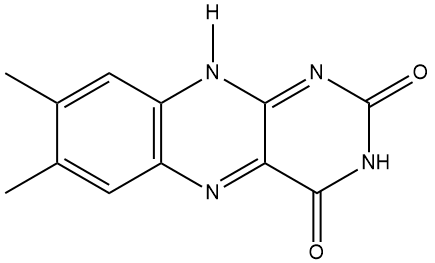 |
| Ribose. The dehydro- version of this is the ‘D’ in DNA. | Isoalloxazine, the building block for the range of organic compounds known as flavins. |
 In 1879, an English chemist by the name of Alexander Wynter Blyth (image, right) isolated a water-soluble material from cow-milk whey that glowed yellowy-green when exposed to light. He called it lactochrome from ‘lacto’ (meaning ‘milk’) and ‘chrome’ meaning ‘colour’. In 1912, Casimir Funk, a Polish biochemist working at the Lister Institute in London, proposed the “vitamine theory”. He had reviewed the work by early scientists studying diseases such as scurvy and beri beri and concluded that these diseases were caused by the deficiency of some vital factor present in food. He believed they were all amine based, so called the vital factors 'vitamines', from vital + amine. Later, this was changed to ‘vitamin’ when many of the molecules were found not to contain an amine group!
In 1879, an English chemist by the name of Alexander Wynter Blyth (image, right) isolated a water-soluble material from cow-milk whey that glowed yellowy-green when exposed to light. He called it lactochrome from ‘lacto’ (meaning ‘milk’) and ‘chrome’ meaning ‘colour’. In 1912, Casimir Funk, a Polish biochemist working at the Lister Institute in London, proposed the “vitamine theory”. He had reviewed the work by early scientists studying diseases such as scurvy and beri beri and concluded that these diseases were caused by the deficiency of some vital factor present in food. He believed they were all amine based, so called the vital factors 'vitamines', from vital + amine. Later, this was changed to ‘vitamin’ when many of the molecules were found not to contain an amine group!
The standard approach to try to identify a vitamin at that time was to first study the effects upon animals (usually lab rats, but sometimes convicts - human prisoners!) of a diet deficient in specific foodstuffs, such as milk, vegetables, or protein. Then add a different food source to the diet one by one until one was found that prevented the deficiency. Finally, isolate and concentrate the particular nutrient (vitamin) in that food and test its potency. This approach had proven particularly successful in finding that citrus fruits prevented scurvy in sailors, and wheat husks prevented beri beri. In the latter case, the vitamin had been identified and called vitamin B (because it was the second vitamin to be identified after vitamin A). But there was a problem – in 1928, Joseph Goldberger and Conrad Elvehjem showed that vitamin B was more than one substance. After it was heated, it was no longer effective in preventing beri beri but it was still prevented stunted growth in rats. Chemists György, Kuhn, and Wagner-Jauregg based in Heidelberg then began the task to try to isolate and identify the various compounds in the vitamin B complex. One group tried to isolate the anti-beriberi compound, while the other concentrated on the factor responsible for rat growth. The task was complicated even further when it was realised that there were 4 compounds in the vitamin B complex, not just 2, and each had a slightly different biological function.
The group trying to isolate the factor responsible for rat growth ran into difficulties, mostly, due to the very low concentrations present in most foods. In fact, the isolation and purification of 1 g of it required more than 5,000 litres of milk or the dried albumin from 34,000 eggs! Eventually, it was isolated as a yellow pigment that they called lactoflavin. This was eventually recognised as the same molecule (lactochrome) discovered earlier by Blyth. Once the structure of lactoflavin had been determined, and the role of the ribose sugar in its structure realised, it was renamed riboflavin.
The factor in vitamin B complex that prevented beri beri was also isolated soon after, and given the name thiamine. When vitamins came to be categorised, the ‘vitamin B’ molecules were subdivided into vitamin B1 (thiamine) and B2 (riboflavin), and the remaining two B3 and B4.
Riboflavin is a coenzyme, which means that it is used as a ‘helper’ molecule by enzymes to perform their physiological process. Because most of these biological processes occur in aqueous environments, versions of riboflavin are required that are more water soluble, such as one with a phosphate chain side-group, flavin mononucleotide (FMN, see above) or one with an adenine side-group called flavin adenine dinucleotide (FAD). FAD can exist in 4 different oxidation states and converted between them by accepting electrons and being reduced, or donating electrons and being oxidised. Its different forms can also accept or donate H+. This huge flexibility in reactions allows FAD to catalyse a large number of important oxidation and reduction reactions in the body.
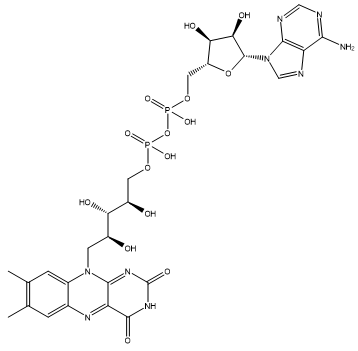 |
| Flavin adenine dinucleotide (FAD) |
There are only a couple of studies on this so far, but the balance of evidence is looking like high doses of riboflavin may, indeed, reduce the severity and frequency of migraines – although more studies need to be performed to confirm this. The side-effect, however, is that too much riboflavin can turn your urine bright yellow!
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5577961]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5577961]