by Philip George, Per E. M.Siegbahn, Jenny P.Glusker and Charles W. Bock,
J. Phys. Chem. B, 1999, 103(35), 7531-7541. - search on eg Coenzyme-B12 and Glusker
Various steps in a mechanism for the diol dehydrase reaction in which a carboxylic acid group of an amino acid residue at the active site of the enzyme serves as an additional cofactor were investigated using density functional theory (B3LYP) calculations. The mechanism examined involved a neutral radical.
With 1,2-dihydroxyethane as the substrate, and formic acid as a model for the carboxylic acid group, the 1,2-dihydroxyeth-l-yl radical (produced by H-atom transfer from the substrate to the 5'-deoxyadenosyl radical) forms a 9-membered ring structure with the formic acid.
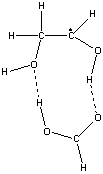 The ring structure has two intermolecular
H-bonds:
The ring structure has two intermolecular
H-bonds:
- with the OH of the HC-OH group in the radical as an H-donor and the C=O group in the formic acid as an acceptor
- with the OH of the H2C-OH group in the radical as an acceptor and the C-OH group of the formic acid as a H-donor
Bond rearrangement within this ring structure results in the formation of an H-bonded product in which transfer of the radical center from one carbon atom to the adjacent carbon atom takes place. Fission of the C-O bond at the new radical center leads to the elimination of H2O and the separation of the formylmethyl radical from which acetaldehyde is then formed by H-atom transfer.
An overall feature of the mechanism is the interchange of the HO-C and C=O bonding in the carboxylic acid group. Like the radical, the substrate diol is found to form a 9-membered ring structure with formic acid, also with two intermolecular H-bonds. This provides support for the hypothesis that two-point attachment of the substrate via its HO groups to the enzyme occurs prior to the H-atom transfer, which initiates the dehydration process.
See Also :
An ab Initio Computational Molecular Orbital Study of Radical, Protonated Radical (Radical Cation), and Carbocation Species That Have Been Proposed in Mechanisms for the Transfer Process in the Enzyme-Coenzyme B12-Catalyzed Dehydration of 1,2-Dihydroxyethane by Philip George, Jenny P. Glusker,* and Charles W. Bock, J. Amer.Chem.Soc., 1997, 119(30), 7065-7074. - search on eg Coenzyme-B12 and Glusker
"The mechanism of action of the enzyme diol dehydrase has variously been suggested to involve a radical, a protonated radical (radical cation), or a carbocation in the apparent transfer of HO from one carbon atom to the other to give acetaldehyde and water. This adenosylcobalamin-requiring B12 enzyme catalyzes dehydration of 1,2-dihydroxyethane. Its active site is buried within a hydrophobic cavity. Each of the three possibilities are examined by ab initio molecular orbital calculations."