Identification of a Rearranged-Substrate, Product Radical Intermediate and the Contribution of a Product Radical Trap in Vitamin B12 Coenzyme-Dependent Ethanolamine Deaminase Catalysis
by Kurt Warncke, Jennifer C. Schmidt, and Shyue-Chu Ke, J.A.C.S.,1999 121, 10522.
"The radical intermediate present during steady-state turnover of substrate aminoethanol by
ethanolamine deaminase from Salmonella typhimurium has been characterized by using X-band electron paramagnetic resonance (EPR) spectroscopy. The radical intermediate was prepared by cryotrapping enzyme, aminoethanol substrate, and vitamin B12 coenzyme (adenosylcob(III)alamin) immediately following mixing. Natural abundance, 1,1,2,2-2H4-, 2-13C-, and 1,2-13C2-aminoethanol were used as substrates. The EPR spectrum obtained for natural abundance aminoethanol shows a broad feature at approximately g = 2.3 that arises from CoII in cob(II)alamin, and a feature from an organic radical that has an absorption maximum at g = 2.02 and a line width of 10.8 mT. The EPR line shape is characteristic of a relatively weakly electron spin-coupled CoII-organic radical system. The EPR line shapes for the 2H- and 13C-labeled substrates were narrowed and broadened by 0.7 and 2.4 mT, respectively, demonstrating that the radical is substrate-based. The comparable line widths of the 2-13C- and 1,2-13C-labeled radicals show that the unpaired spin density is localized primarily at the C2 carbon atom. This identifies the radical intermediate as a rearranged substrate radical, or product radical. The results are consistent with either the 1-aminoethanol-2-yl radical or the ethanal-2-yl radical, which have been proposed as intermediates in, respectively, the amine migration and amine elimination mechanisms of rearrangement. A qualitative reaction free energy profile for the CoII-radical pair intermediate states on the enzyme is constructed, based on the EPR results and previous isotope exchange and kinetic isotope effect studies. The results and analysis reveal that a product radical trap strategy contributes to the stabilization of the radical pair state, which enhances catalytic performance of ethanolamine deaminase."
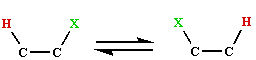 is considered to take place by way of a radical mechanism. The evidence for this comes principally from observations of EMR spectra for several of the active enzyme systems. Thus if the dioldehydrase (holoenzyme) is treated with the substrate analogue 1,2-propanediol, a radical signal believed to be due to the 5'-deoxyadenosyl radical, together with a low spin Co(II) signal is formed within milliseconds, and persists whilst there is substrate present . Similarly for glycerol-dehydrase, ethanol-ammonia lyase and the ribonucleotide reductase system.
is considered to take place by way of a radical mechanism. The evidence for this comes principally from observations of EMR spectra for several of the active enzyme systems. Thus if the dioldehydrase (holoenzyme) is treated with the substrate analogue 1,2-propanediol, a radical signal believed to be due to the 5'-deoxyadenosyl radical, together with a low spin Co(II) signal is formed within milliseconds, and persists whilst there is substrate present . Similarly for glycerol-dehydrase, ethanol-ammonia lyase and the ribonucleotide reductase system.
 - and magnetic field-dependent five-line pattern of narrow features. This indicates that the substrate and product radicals are comparably coupled to the same 14N nucleus. ESEEM simulations give the following best-fit principal hyperfine tensor and nuclear quadrupole coupling parameters: A = [-1.1, -0.8, -0.8] MHz; e2qQ/h = 3.05 MHz;
- and magnetic field-dependent five-line pattern of narrow features. This indicates that the substrate and product radicals are comparably coupled to the same 14N nucleus. ESEEM simulations give the following best-fit principal hyperfine tensor and nuclear quadrupole coupling parameters: A = [-1.1, -0.8, -0.8] MHz; e2qQ/h = 3.05 MHz;  = 0.51. The quadrupole parameters are consistent with a secondary amide nitrogen in a polypeptide bond or with the N10 amino nitrogen in the adenine ring of 5'-deoxyadenosine. The equivalent 14N coupling for each radical indicates that either the nitrogen nucleus lies along the C1-C2 bond bisector or the interaction responsible for the coupling moves to remain in register with the center of unpaired spin density as it shifts 1.5 Å from C1 to C2 in the substrate-to-product radical transformation. The longer separation distance from CoII of the product versus substrate radical indicates that the principle of radical pair stabilization by separation operates continuously through the rearrangement reaction.
= 0.51. The quadrupole parameters are consistent with a secondary amide nitrogen in a polypeptide bond or with the N10 amino nitrogen in the adenine ring of 5'-deoxyadenosine. The equivalent 14N coupling for each radical indicates that either the nitrogen nucleus lies along the C1-C2 bond bisector or the interaction responsible for the coupling moves to remain in register with the center of unpaired spin density as it shifts 1.5 Å from C1 to C2 in the substrate-to-product radical transformation. The longer separation distance from CoII of the product versus substrate radical indicates that the principle of radical pair stabilization by separation operates continuously through the rearrangement reaction.