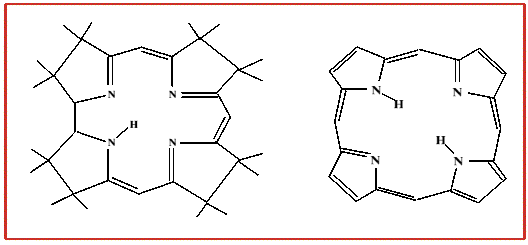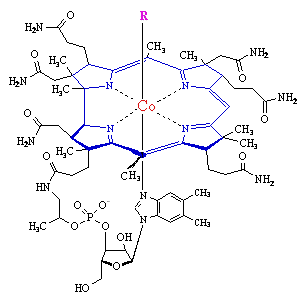
- a methyl group - as in methylcobalamin
- a 5'-deoxyadenosine at the the 5' positon - as in adenosylcobalamin (coenzyme B12
- a cyanide group - as in Vitamin B12 - as supplied from drug companies
A methyl-nickel intermediate on acetyl-CoA synthase is also known, but only as an intermediate rather than a stable, isolable compound as the three cobalamins. Other organometals such as the methylmercury ion are highly toxic, it is interesting that there is an unfortunate connection between CH3Hg+ and methylcobalamin.