B12 Models with Highly Distorted
N4 Equatorial Ligation and a Co-C-N Ring:
Structural Assessment of the Steric Influence of
Benzimidazole and Imidazole Axial Ligands.
by Luigi G Marzilli, Suzette M Polson,. Lory Hansen, Scott J
Moore,. Patricia A Marzilli Inorg. Chem. 1997,
36(18), 3854-3860.
Abstract
In human B12 enzymes, a histidyl imidazole is the
lower axial ligand instead of the benzimidazole of the
coenzymes. The differences in the binding interactions of
these ligands have been examined using a novel class of
organocobalt models, [LCo(N-CH2-chel)]X (I), with
an unusually spacious lower coordination site created by a
rare Co-C-N ring. The work compares two new analogs, 1
(L = 1,5,6-trimethylbenzimidazole = Me3Bzm) and
2 (L = N-methylimidazole = N-MeImd), with the simple
analog, 3 (L = py).
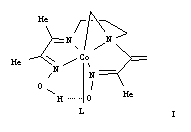
The three structures (X = PF6 for 1 and
2; X = ClO4 for 3) have similar
geometrical parameters for the ring atoms (N(2), Co, C(12)). A
pocket under the Co-C-N group is created by the raised position
of N(2) above the plane of the other three equatorial N donors,
the cis oxime N, N(1), the trans oxime N, N(4),
and the cis imine N, N(3). A net upward bending is
clearly shown by the sum of the four cis N-Co-N bond
angles involving the L ligating atom, N(5). The sum is
approximately 23° more in the new models than in related
imine/oxime-type (I/O) models. The distortions around N(5)
differ significantly for the three structures. The Co-N(5) bond
of Me3Bzm complex 1 is tilted furthest away
from the Co-C-N pocket, and the N(5)-Co-N(2) angle is 111°.
The value of the N(5)-Co-N(4) angle (96°) is close to that
of the related angle (95°) in the I/O model,
[Me3BzmCo((DO)(DOH)pn)CH3]PF6.
In contrast, the N(5)-Co-N(4) angle of the N-MeImd and the py
complexes, 2 and 3, is larger than that in I/O
complexes, suggesting that these ligands are small enough to
move toward the pocket. These and other structural parameters
suggest clear differences between the steric interactions of
the equatorial ligand with the imidazole and with the
benzimidazole ligands. These complexes have unusual
1H NMR properties, e.g. a large remote
isotope effect on some CH signals after exchange of the oxime
OH to OD. At pH 13, the N4C chelate of
[H2OCo(N-CH2-chel)]+ reverts,
in part, to the classical I/O N4 chelate, suggesting a stepwise
mechanism involving C-N bond cleavage to form a
Co-CH2OH intermediate, which then undergoes
base-catalyzed Co-C bond cleavage

