Ha ha! By 'Ginger Spice', you mean a molecule you get from ginger?
Yes, of course.
But what actually is it?
The textbooks will tell you that zingiberene is a monocyclic sesquiterpene.
Translate, please
Monocyclic means that its structure contains a single ring. Terpenes are hydrocarbons whose formulae are multiples of the formula of isoprene, 2-methyl-1,3-butadiene (MOTM July 2008). Monoterpenes have two isoprene units joined together; sesquiterpenes have three; diterpenes have four, and so on. Isoprene is C5H8, so terpenes are (C5H8)n (n = 2,3,4 etc.). For sesquiterpenes, n = 3, so they have the formula C15H24.
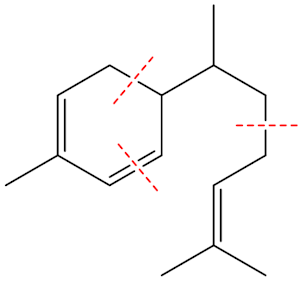
How 3 isoprene molecules combine to form zingiberene
So it is made by joining three isoprene molecules together?
Only on paper. The process in real life involves the “isoprenoid pathway”, starting from farnesylpyrophosphate. Free isoprene molecules are not involved, but the end result is the same.
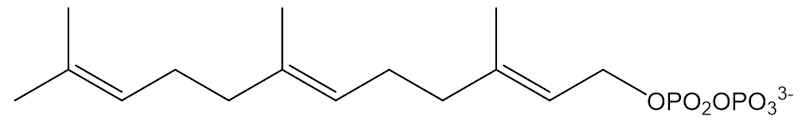
Farnesyl pyrophosphate
 Why is zingiberene important?
Why is zingiberene important?
It is the most abundant molecule in oil of ginger (Zingiber officinale), comprising 30% or more of the oil (photo, right). It occurs in a whole range of plant species, including tomato, basil, turmeric, cardamom and sorghum, too.
Why do plants make it?
Very likely to defend them against insect pests, fulfilling roles that might include stopping insects from laying eggs and deterring them from feeding on the plant. Zingiberene has been shown to confer resistance to insect pests upon tomatoes.
And zingiberene is responsible for the flavour of ginger?
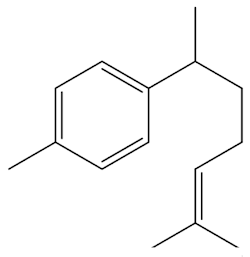
It is one of the main volatiles, along with molecules like nerolidol, geraniol and curcumene.
 |
 |
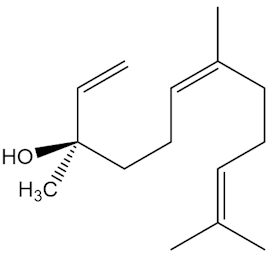 |
| Curcumene |
Geraniol |
Nerolidol |
 So it puts the ‘zing’ into ginger?
So it puts the ‘zing’ into ginger?
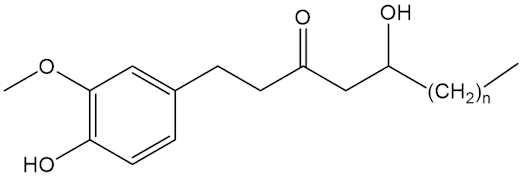
No, that is down to molecules like the shogaols, the gingerols and zingerone.
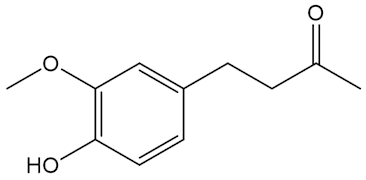 |
 |
| Zingerone |
Gingerols n=4,6,8 |
| |
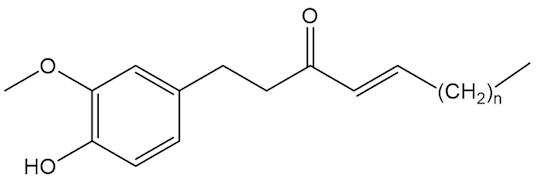 |
|
Shogaols n=4,6,8 |
OK, where does this Epizingiberene stuff come in?
It’s the epimer of zingiberene.
Sounds like a sort of isomer
Yes, epimers are diastereoisomers that only differ in the configuration at one stereogenic (chiral) centre. To put it another way, they are molecules with a number of chiral centres that are identical, apart from one centre. Zingiberene has the 4S, 7S stereochemistry; epizingiberene is the 4S, 7R isomer.
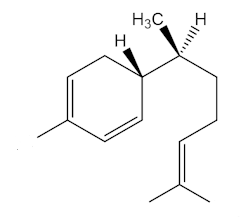 |
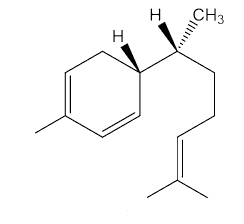 |
| Zingiberene |
Epizingiberene |
Another example of epimers is testosterone and epitestosterone.
Are they the same?
A study of wild tomatoes (Solanum habrochaites) has shown that unlike cultivated tomatoes (Solanum lycopersicum) they emit epizingiberene, and this confers superior herbivore resistance – it is toxic to a key pest, the sweet potato whitefly (Bemisia tabaci), in particular. A study concluded that, along with R-curcumene, they “apparently repel adult whiteflies prior to landing, presumably because it informs them that after landing they, or their offspring, may be exposed to higher and lethal concentrations of the same compounds.” Evidently, during cultivation the gene that produces epizingiberene was lost, doubtless through concentrating the breeding on producing tomatoes with larger, redder fruit. Scientists are studying ways of introducing this genetic pathway into cultivated tomatoes to improve yields.
 |
 |
Wild tomatoes
(Solanum habrochaites) | The sweet potato whitefly
(Bemisia tabaci) |
Why does it matter that the epimer is produced by the tomato?
The fact that zingiberene has no effect but epizingiberene is effective comes down to a receptor binding site having a specific shape that can dock epizingiberene molecules but not zingiberene. Nature really is that picky.
Apart from being a useful food additive, does ginger have any uses?
Ginger is supposed to be effective against motion sickness, whilst it is being investigated to see if it can reduce chemotherapy-induced nausea. Zigang Dong and fellow researchers at the University of Minnesota have shown that [6]-gingerol, an important spicy molecule in ginger that is a precursor for zingerone, suppresses colonic cancer growth by targeting an enzyme Leukotriene A4 Hydrolase. The same molecule has been found to be active in suppressing skin tumours in mice and it shows activity against ovarian cancer cells and breast cancer cells. Of course, these are a long way from being a cure for anything.
![[6]-gingerol - click for 3D structure [6]-gingerol](6-gingerol.gif) |
| [6]-Gingerol |

Bibliography
- Chapman and Hall Combined Chemical Dictionary compound code numbers: JXX90-J (props, bibliography of zingiberene); MSJ77-Q (epizingiberene).
Ginger
- G. Vernin and C. Párkányi, in G. Charalambous ed., Spices, herbs and edible fungi, Elsevier, Amsterdam, 1994, pp 579-594 (ginger oil)
- T. J. Zachariah, in V.A. Parthasarathy, B. Chempakam and T. J. Zachariah eds., Chemistry of Spices, CABI International, Wallingford, 2008, pp 70-96 (ginger)
- Z. Kamaliroostaa, L. Kamaliroosta and A. H. Elhamirad, J. Food Biosci. and Technol., 2013, 3, 73-80 (ginger oil)
Zingiberene and epizingiberene
- A. Eschenmoser and H. Schinz, Helv. Chim. Acta, 1950, 33, 171-177 (isolation, IR spectrum, structure)
- D. Arigoni and O. Jeger, Helv. Chim. Acta, 1954, 37, 881-883 (absolute configuration of zingiberene)
- M. D. Soffer and L. A . Burk, Tet. Lett., 1985, 26, 3543-3546 (crystal structure)
- J. G. Millar, J. Nat. Prod., 1998, 61, 1025–1026 (isolation from ginger essential oil)
- M. Butu, M. Butariu, S. Rodrino and A. Butu, Dig. J. Nanomat. Biosci., 2014, 9, 935–941 (Mass Spectrum of zingiberene)
- D. C. Breeden and R. M. Coates, Tetrahedron, 1994, 50, 11123–11132 (isolation of epizingiberene from wild tomato leaves)
Biosynthesis of zingiberene and epizingiberene
- http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/terp/zingiber.html (zingiberene)
- http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/terp/epizing.html (epizingiberene)
- K. Rani, Fitoterapia, 1999, 70, 568-574 (zingiberene biosynthesis)
- E. Gonzales-Vigil, D. E. Hufnagel, J. Kim, R. L. Last and C. S. Barry, Plant J., 2012, 71, 921–935 (terpene synthases that make epizingiberene)
Zingiberene and resistance to pests
- W. R. Maluf, G. A. Campos and M. G. Cardoso, Euphytica, 2001, 121, 73–80 (resistance of tomato to spider mites)
- J.A. Freitas, W. R. Maluf, M. Graças-Cardoso, L.A.A. Gomes and E. Bearzotti, Euphytica, 2002, 127, 275–287. (resistance of tomato to whitefly)
- S. M. de Azevedo, M. V. Faria, W. R. Maluf, A. C. B. de Oliveira and J. A. de Freitas,
Euphytica, 2003, 134, 347–351. (resistance of tomato to tomato pinworm)
- P. M. Bleeker, P. J. Diergaarde, K. Ament, J. Guerra, M. Weidner, S. Schütz, M. T. J. de Both, M. A. Haring, and R. C. Schuurink, Plant Physiology, 2009, 151, 925–935 (repelling whitefly)
Epizingiberene and resistance to pests
- G. F. Antonious and T. S. Kochhar, J. Environ. Sci. Health A, 2003, 38, 489-500 (zingiberene in wild tomato)
- P. M. Bleeker, P. J. Diergaarde, K. Ament, S. Schütz, B. Johne, J. Dijkink, H. Hiemstra, R.de Gelder, M. T. J. de Both, M. W. Sabelis, M. A. Haring and R. C. Schuurink, Phytochem., 2011, 72, 68–73 (epizingiberene in wild tomato as repellent to whiteflies)
- P. M. Bleeker, R. Mirabella, P. J. Diergaarde, A. VanDoorn, A. Tissier, M. R. Kant, M. Prins, M. de Vos, M. A. Haring and R. C. Schuurink, Proc. Natl. Acad. Sci. USA, 2012, 49, 20124–20129
(introduction of epizingiberene into cultivated tomatoes improves herbivore resistance)
Medicinal uses of ginger
- K.-K. Park, K.-S. Chun, J.-M.Lee, S. S. Lee and Y.-J. Surh, Cancer Lett., 1998, 129, 139–144 (gingerol and skin tumours in mice)
- H.-C. Lien , W. M. Sun , Y.-H. Chen, H. Kim , W. Hasler , C. Owyang, Am. J. Physiol. Gastrointest. Liver Physiol., 2003, 284, G481–G489 (ginger and motion sickness)
- Y. Shukla and M. Singh, Food Chem. Toxicol., 2007, 45, 683–690 (review of cancer preventive properties of ginger)
- J. Rhode, S. Fogoros, S. Zick, H. Wahl, K. A. Griffith, J. Huang and J. R. Liu, BMC Compl. and Alt. Med. 2007, 7, 44 (ginger and ovarian cancer cell growth)
- C.-H. Jeong, A. M. Bode, A. Pugliese, Y.-Y. Cho, H.-G. Kim, J.-H. Shim, Y.-J. Jeon, H. Li, H. Jiang and Z. Dong, Cancer Res., 2009, 69, 5584-5591 (ginger and colonic cancer)
- A. I. Elkady, O. A. Abuzinadah, N. A. Baeshen and T. R. Rahmy, J. Biomed. Biotechnol., 2012, Article ID 614356. (activity against breast cancer cells).
- J. Lee and H. Oh, Oncology Nursing Forum, 2013, 40, 163-170 (review, ginger as an antiemetic)
- W. M. Marx, L. Teleni, A. L. McCarthy, L. Vitetta, D. McKavanagh, D.Thomson and E. Isenring, Nutrition Rev., 2013, 71, 245–254 (review, ginger as an antiemetic)
- W. Marx, A. L. McCarthy, K. Ried, L. Vitetta, D. McKavanagh, D. Thomson, A. Sali and L. Isenring, BMC Compl. and Alt. Med., 2014, 14, 134 (ginger as possible treatment for chemotherapy-induced nausea)
- http://www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/herbsvitaminsandminerals/ginger (American Cancer Society’s site)


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5504017]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5504017]

![]()
![]()
![]()
![]()
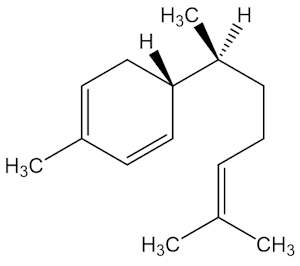



 Why is zingiberene important?
Why is zingiberene important?


 So it puts the ‘zing’ into ginger?
So it puts the ‘zing’ into ginger?






![[6]-gingerol - click for 3D structure [6]-gingerol](6-gingerol.gif)
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5504017]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5504017]