 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
|
|
||||||
| Anion Recognition and Transport | ||||||
| To view in 3D requires MDL's Chime Plugin | ||||||
| Download Now | ||||||
|
|
||||||
| Cholic acid is a readily available natural product with a rigid, polycyclic steroidal structure and a well-spaced array of differentiable functional groups. It makes an excellent starting material for functionalised molecular architectures with well-defined geometrical properties. We have used it in many of our research programmes (see elsewhere on this site), but especially for the construction of anion receptors with preorganised arrays of H-bond donor groups. |
|
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
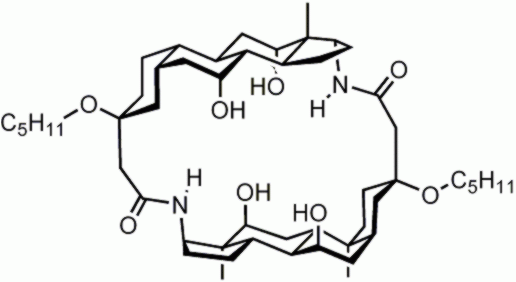
In this early example, two
steroids were used to create a cavity surrounded by 4 x OH and 2 x NH groups. The molecule bound
chloride and fluoride with moderate affinities. See: A. P. Davis, J. F. Gilmer, J. J. Perry, Angew. Chem. Int. Ed. Engl. 1996, 35, 1312. |
 |
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
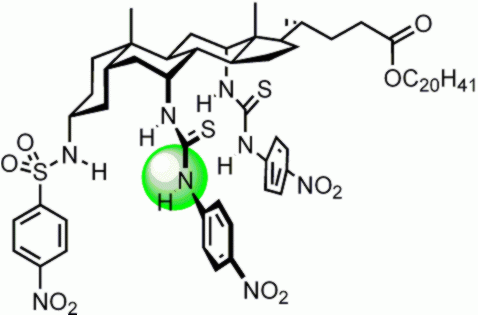
More recently, we have adopted the “cholapod” architecture, based on a single cholic acid unit with three “legs”. The legs can contain various numbers of H-bond donors, and these can be designed to be very powerful. This example binds chloride with Ka ~ 1011 m-1 in chloroform. For more details see: J. P. Clare et al., J. Am. Chem. Soc. 2005, 127, 10739 and A. P. Davis, Coord. Chem. Rev. 2006, 250, 2939. |
 |
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
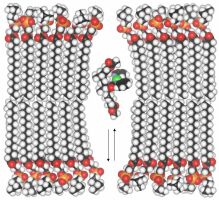
The cholapods' unusual
binding strength
enables them to transport certain anions (especially chloride) across cell
membranes. It seems that they can extract the anion from water into the
non-polar membrane interior, without transferring a counter-cation. The
anion is then carried across and released on the other side of the membrane.
The net effect is the same as a natural chloride channel. Certain
diseases, notably cystic fibrosis, occur because sufferers possess defective
chloride channels. Could cholapods replace the missing function in these
patients? For more, see: A. V. Koulov et al., Angew. Chem. Int. Ed. 2003, 42, 4931 and A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev. 2007, 36, 348. |
 |
|||||
|
|
||||||