 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
|
|
||||||
| Carbohydrate Recognition | ||||||
| To view in 3D requires MDL's Chime Plugin | ||||||
| Download Now | ||||||
|
|
||||||
|
Carbohydrates are
interesting and challenging targets for supramolecular chemistry. They are
biologically important, their structures are complex, and they are very happy to
remain in water (where they are usually found). In early work, we used an
inexpensive steroidal starting material, cholic
acid, to create cavities with inward-directed polar functional groups.
These “cholaphanes” were able to bind lipophilic glycosides in chloroform, but
could not compete with water.
For an overview of this work, see: A. P. Davis and R. S. Wareham, Angew. Chem. Int. Ed. 1999, 38, 2978. |
 |
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
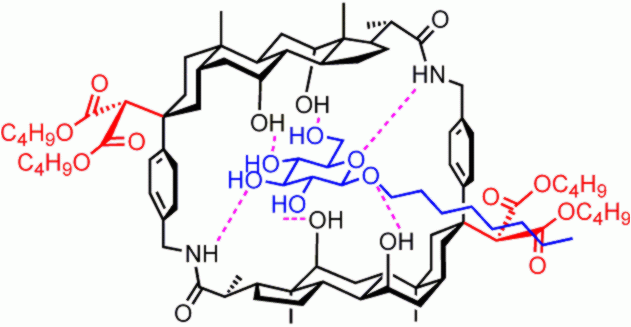
More recently we have
developed polycyclic architectures which are specifically designed to bind
"all-equatorial" carbohydrates (e.g. b-glucosides).
These receptors possess cavities with a hydrophobic "roof" and "floor",
separated by polar spacers. The substrate's equatorial polar groups are
bound through H-bonding, while the axially-directed CH make contact with the hydrophobic surfaces. In
one system (right), aimed at monosaccharides, the hydrophobic surfaces are
biphenyl units. The system is intrinsically more powerful and, with the solubilising groups
shown, can be studied in water. This receptor does bind glucose in water,
albeit weakly, and shows the expected selectivity for equatorial substituents.
Now that we can bind carbohydrates in their natural environment, it is realistic
to consider applications. For example, a glucose-selective receptor could
be used in a new type of glucose sensor, potentially useful for diabetics.
For more details see: E. Klein et al. Angew. Chem. Int. Ed. 2005, 44, 298. |
 |
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
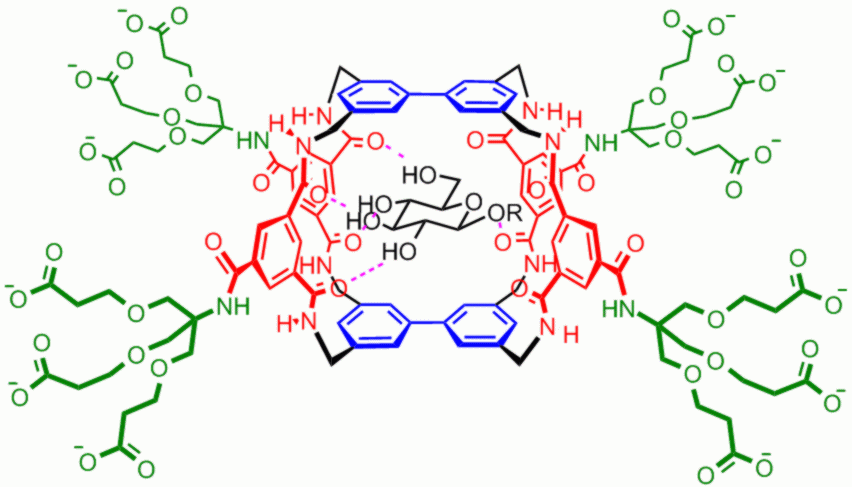
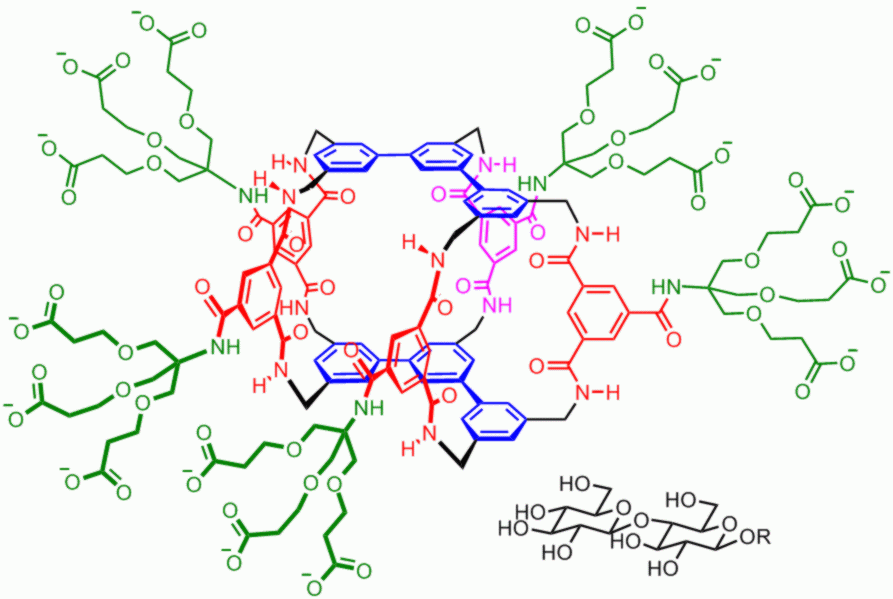
Carbohydrates in nature are often combined into oligosaccharides, then used as labels for proteins and cells. The labels are “read” by proteins called lectins. By extending our monosaccharide receptor (see above), we have made a “synthetic lectin” for the all-equatorial disaccharide cellobiose. The terphenyl-based system binds cellobiose quite strongly in water, and shows remarkable selectivity. Most other carbohydrates are ignored, and the cellobiose complex can form even in the presence of 8 competing substrates. For more details see: Y. Ferrand, M. P. Crump, A. P. Davis, Science 2007, 318, 619. |
 |
|||||
| click to view 3D rendering | ||||||
|
|
||||||