 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
 |
||||||||||||||||
| School of Chemistry, University of Bristol, Bristol BS8 1TS, UK - Tel + 44 (0)117 95 46 334 - Fax + 44 (0)117 92 51 295 | ||||||||||||||||
|
|
email: Anthony.Davis@bristol.ac.uk | |||||||||||||||
|
|
||||||
| Cholic Acid in Supramolecular Chemistry | ||||||
| To view in 3D requires MDL's Chime Plugin | ||||||
| Download Now | ||||||
|
|
||||||
| As shown on other pages, the exploitation of cholic acid is a major theme of our work. This large, rigid, inexpensive molecule makes an excellent starting material for functional molecules. At present, our main uses for cholic acid lie in the areas of anion recognition/transport and crystal engineering (see elsewhere), but there are many other possibilities. Some of our past efforts are described below, and it is likely we will explore further applications in the future. |
|
|||||
| click to view 3D rendering | ||||||
|
|
||||||
|
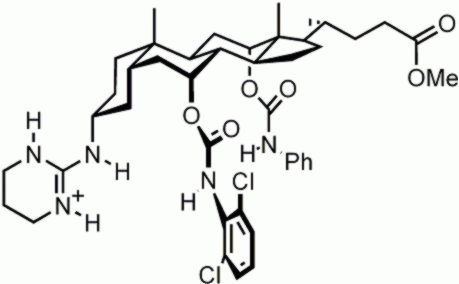
Enantioselective
receptors. Cholic acid is chiral, and
therefore well-suited as a starting material for enantioselective receptors.
This cholapod was designed to extract N-acyl amino acid carboxylates from
water, through salt-bridge formation with the guanidinium group. It was capable
of binding the L-enantiomers with up to 80% enantiomeric excess. For more details see: L. J. Lawless et al., J. Chem. Soc. Perkin Trans. 1 2001, 1329. |
 |
|||||
|
|
||||||
|
Facial amphiphiles. This tris-ammonium cation has a lipophilic face and a highly polar face. It was found to promote the fusion of vesicles (“artificial cells”), and also to carry DNA into living cells. For details see: Y. R. Vandenburg et al., J. Am. Chem. Soc. 2000, 122, 3252. |
|
|||||
|
|
||||||
|
Scaffolds
for combinatorial chemistry.
Combinatorial chemistry allows one to produce large libraries of compounds very
quickly, provided straightforward, high-yielding reactions can be used. Amine derivatisations are especially easy, so amino-substituted “scaffolds” make good
starting points. However the amino groups must be “masked” so that they can be
revealed one by one. We have shown that cholic acid can be converted into
differentially-protected polyamines (e.g. right), and that these scaffolds can
be converted into libraries which may contain enzyme-like catalysts. For more details see: H. De Muynck et al., Angew. Chem. Int. Ed. 2000, 39, 145. V. del Amo et al., Org. Biomol. Chem. 2004, 2, 3320. |
|
|||||
|
|
||||||