| |
|
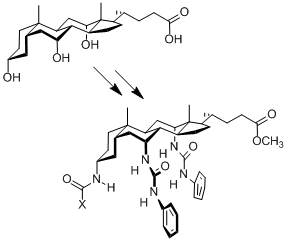
The design of crystals with
predictable packing arrangements and properties is a major challenge for
supramolecular chemistry. We entered this area by accident, through our
work on anion receptors/transporters. We transformed cholic acid into a
number of bis-phenylureas, and found that some of them formed needle-like
crystals (see right; X = CF3, NHPh, OBut). When we
determined the crystal structures we found that the packings were almost
identical, and quite remarkable.... |
|
 |
|
|
| |
|
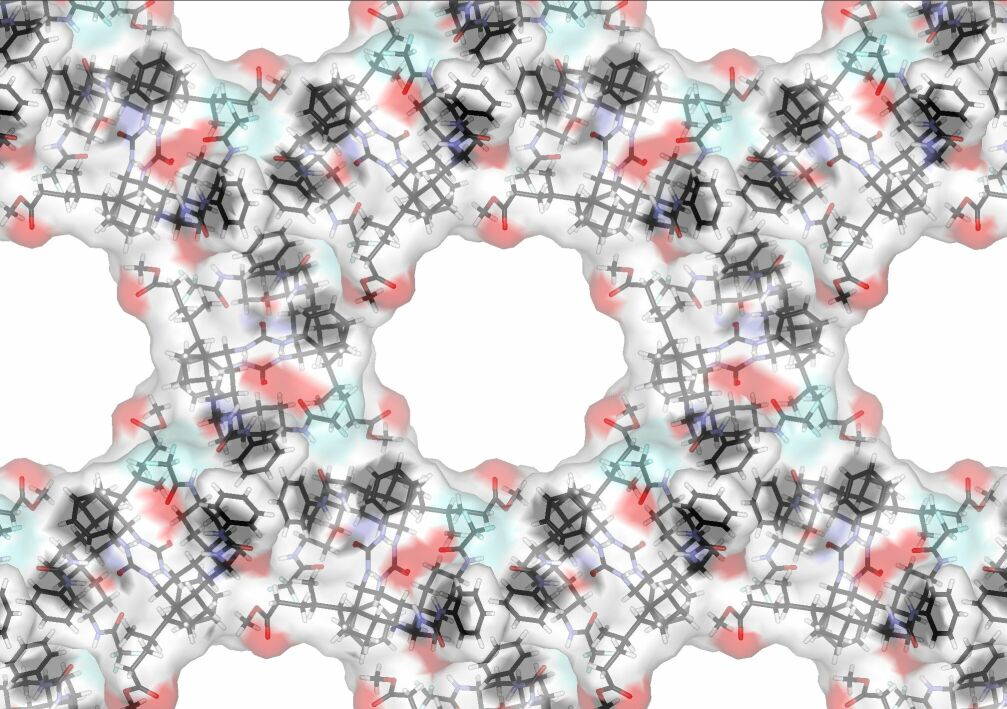
The molecules arrange themselves
in a honeycomb pattern with broad channels running through the crystals.
The picture (right) shows the structure for X = CF3, viewed along the
length of the channels. The gaps are originally filled with solvent, but
these can be replaced by other molecules. As the channels are so wide (up
to 1.4 nm) they could in principle accommodate a great variety of guests.
The group X points into the channel, so it is possible to change this unit
without disrupting the structure. This means we can tune the size and
nature of the channels, and perhaps design crystals for specific purposes.
Such "nanoporous" crystals have potential for separations, catalysis and
optoelectronic applications.
For more details see: A. L. Sisson et al.,
Angew. Chem. Int. Ed. 2005, 44, 6878. |
|
 |
|
|



