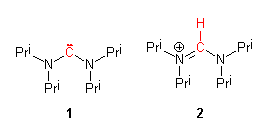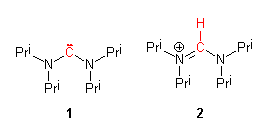
Bis(diisopropylamino)carbene
Roger W. Alder,* Paul R. Allen, Martin Murray, and A. Guy Orpen, accepted for publication in Angew. Chem. Int. Ed. Engl.
We report the isolation of bis(diisopropylamino)carbene (N,N,N',N'-tetraisopropylformamidinylidene) 1 as a stable solid by deprotonation of N,N,N',N'-tetraisopropylformamidinium chloride 2 with lithium diisopropylamide (LDA) in THF. The X-ray crystal structure of 1 was determined from a crystal grown by sublimation, and shows a much larger N-C-N angle than any previously studied diaminocarbene derivative (121.0(5) degrees). Variable temperature NMR studies of 1 yield a barrier for rotation about the bonds to the carbene carbon of 53 kJ/mol, almost the same as for the formamidinium ion precursor 2 (55 kJ/mol).