Professor Chris Willis – Research Summary
Natural products isolated from bacteria, fungi and plants from both marine and terrestrial environments are a rich source of compounds of medicinal and agrochemical importance. We have published >100 papers which centre upon:-
- Structure elucidation and total synthesis of natural products
- Design and synthesis of probes to elucidate biosynthetic pathways and enzyme mechanisms
- Enantioselective synthesis of amino acids and heterocycles
- Isotopic labelling, biotransformations
and reaction mechanisms
Research programmes
Total syntheses of a number of natural products have been completed (for example those shown below) and new targets being pursued include those with anticancer and antibiotic properties.
Prins
cyclisations: Mechanism and applications in natural product synthesis
The stereocontrolled synthesis of
oxygen heterocycles is an important goal and our interest in this area stems
from the wealth of structural diversity of marine natural products assembled on
a tetrahydropyran core. Prins-type cyclisations have the potential to give
versatile and efficient approaches to the synthesis of substituted tetrahydropyrans.
In collaboration with Professor Roger Alder we are investigating the mechanism
of Prins cyclisations and applying the chemistry in natural product synthesis. Studies in this area are ongoing and recent
results have revealed that:-
- The mechanism of Prins cyclisations is not simple and can involve fragmentations, allyl transfer processes and 2-oxonia Cope rearrangements.
- By varying the reaction conditions halogen, oxygen
and nitrogen nucleophiles may be introduced at C-4 of the
tetrahydropyrans.
- A deeper understanding of the reaction mechanism
has facilitated the first total synthesis of 2 catechol natural products
isolated from Plectranthus
sylvestris (labiatae).
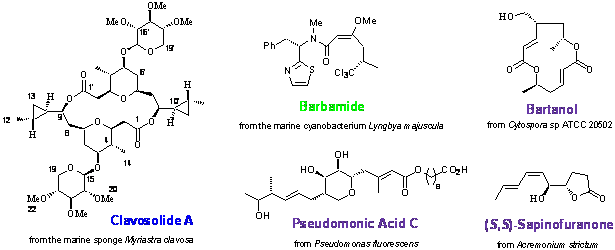
- Reaction of aldehydes with homoallylic alcohols can give tetrahydropyrans in excellent yields and with the creation of 3 new asymmetric centres with complete stereocontrol in a single step. The chemistry has been applied to the total synthesis of the 16-membered ring dilactone, clavosolide A isolated from a marine sponge which revealed that the original structure proposed in the literature was a diastereomer of the natural product.

Selected publications:
Total synthesis of a diastereomer of the marine natural product clavosolide A, Chem. Comm., 2005, 5097.
Stereoselective synthesis of the tetrahydropyran core of polycavernoside A, Org. Lett., 2005, 7, 2683.
Probing the mechanism of Prins cyclisations and application to the synthesis of 4-hydroxytetra-hydropyrans, Chem. Comm., 2005, 3727.
Stereoselective synthesis of 4-hydroxy-2,3,6-trisubstituted tetrahydropyrans, Org. Lett., 2003, 5, 2429.
Prins cyclisations: labelling
studies and applications to natural product synthesis, Org. Lett., 2002, 4, 3407.
Oxonia-Cope rearrangement and side-chain exchange in the Prins cyclisation, Org. Lett, 2002, 4, 577.
Stereocontrolled synthesis of 2,4,5-trisubstituted tetrahydropyrans, Chem. Commun., 2001, 835.
Chlorinated
marine natural products
The marine environment is proving
to be a treasure trove of biologically active molecules and some of these
secondary metabolites are emerging as lead compounds in drug discovery. A
fascinating structural feature of many marine natural products is the covalent
inclusion of halides. In collaboration
with Professor Bill Gerwick,
- The first total synthesis of barbamide.
- Synthesis and incubation studies with novel amino
acids selectively labelled with carbon-13
have revealed that selective chlorination of the unactivated pro-R methyl group of leucine
occurs. With no apparent activation we propose that a novel biochemical
stepwise mechanism of chlorination operates, possibly involving radicals.
- The gene cluster responsible for the biosynthesis
of barbamide has been cloned and characterised.

Selected publications:
[6-13C]-(2S,4S)-5-Chloroleucine: Synthesis and incubation studies with cultures of the cyanobacterium, Lynbya majuscula, Tetrahedron Lett., 2003, 44, 285.
The barbamide biosynthetic gene cluster: a novel marine cyanobacterial system of mixed polyketide (PKS)-non-ribosomal peptide synthase (NRPS) origin involving an unusual trichloromethyl starter unit, Gene, 2002, 296, 235.
Barbamide and dechlorobarbamide, molluscicidal agents from the marine cyanobacterium, Lyngbya majuscula: Biosynthetic pathway and origin of the chlorinated methyl group, Tetrahedron, 2000, 56, 9103.
Total synthesis of the marine natural product barbamide, Chem. Commun., 2001, 1934.
Biosynthesis of the marine cyanobacterial metabolite barbamide. 1. Origin of the trichloromethyl group, J. Am. Chem. Soc., 1998, 120, 7131.
Polyketide
derived natural products
The study of polyketides remains
an area of intense research interest worldwide.
The scope of the biosynthetic pathways for exploitation to make new
biologically active entities via emerging methods of combinatorial biosynthesis
are virtually limitless. In collaboration with Professor Tom Simpson, Drs
Russell Cox, John Crosby and Matt Crump in Bristol as well as Professor Chris
Thomas and his group at the University of Birmingham, investigations
(synthetic, biosynthetic and genetic) are targeted at a range of polyketide
derived natural products including the pseudomonic acids, monocerin and the decarestrictines.
Research in our laboratories has led to:-
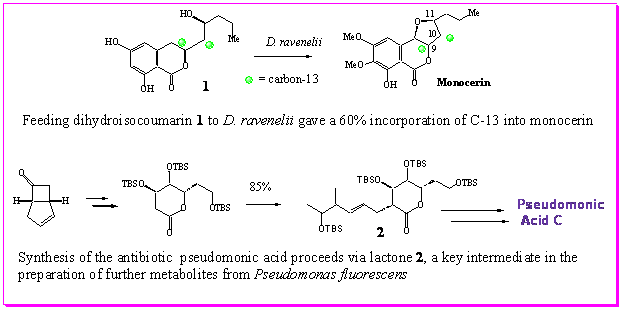
- The total synthesis of pseudomonic acid C (an
antibiotic isolated from Pseudomonas
fluorescens) by a versatile approach which may be readily adapted for
the synthesis of advanced biosynthetic
intermediates.
- Use of spectroscopic methods for the elucidation of
the structure of two unusual 13-membered ring macrolides isolated from Cytopsora and confirmation of the
structure of bartanol through total synthesis.
- Elucidation of the structure of a novel g-lactone metabolite, (S,S)-sapinofuranone, from Acremonium
strictum and confirmation of the structure by total synthesis.
- Through synthesis and feeding studies we have
provided compelling evidence for the structure of the first enzyme-free
intermediate on the biosynthetic pathway to monocerin. This work has paved the way for use of
this intermediate in further studies to isolate and sequence the moncerin
PKS gene.
- Methods have been developed for the enantioselective synthesis of a range of putative biosynthetic intermediates to polyketides which have been used to gain a deeper understanding of the assembly of polyketides.
Selected publications:-
Assembly intermediates in polyketide biosynthesis: Enantioselective syntheses of b-hydroxycarbonyl compounds, Org. Biomol. Chem., 2005, 3, 1719.
Synthesis and incorporation of the first PKS-free intermediate in monocerin biosynthesis, Angew. Chem., Int. Ed, 2004, 727.
Two approaches to the synthesis of the macrodiolide colletotriene, Aust. J. Chem., 2004, 645.
A versatile approach to the total synthesis of the pseudomonic acids, Chem. Commun., 2000, 1109.
Structure elucidation and synthesis of (4S,5S,6Z,8E)-5-hydroxydeca-6,8-dien-4-olide (S,S, Sapinofuranone B) - a novel g-lactone metabolite of Acremonium strictum, J. Chem. Soc., Perkin Trans. 1, 2000, 2475.
Isotopic
labelling and biotransformations
Biotransformations are of widespread value in asymmetric
synthesis and we are developing new methods for the stereocontrolled synthesis
of molecules of biological interest such as substituted piperidines, pyrrolidines
and isotopically labelled amino acids using enzymes in key synthetic
transformations. For example:-
- Combining two biotransformations in a single pot
process has given a clean and efficient method for the conversion of 2-oxo
esters to enantiomerically pure a-amino
acids and 2-hydroxy acids. The approaches have been adapted for the
selective incorporation of isotopic labels and the products used to probe
biochemical mechanisms and protein structure.
- Genetic manipulation of oxidoreductases has given
catalysts with exciting potential in organic synthesis not only due to
their broad substrate specificity but also their high catalytic activity.
- Oxidoreductases have been shown to catalyse a
combined resolution-reduction which we are exploiting for use in organic synthesis
as well as to further probe the active sites of these enzymes.
- We have reported the first examples of the
reduction of 2-oxo acids with nitrogen functionality in the side chain giving
valuable intermediates for the enantioselective synthesis of a series of
5- and 6- membered ring nitrogen heterocycles.
- Isotopic labelling studies on the Ugi four component reaction have led to a greater understanding of the mechanism and utility of the reaction in organic synthesis.

Selected publications:-
Three approaches to the synthesis of L-leucine selectively labelled with carbon-13 or deuterium in either diastereotopic methyl group, J. Chem. Soc., Perkin Trans. 1, 2000, 43.
Chemoenzymatic syntheses of cis- and trans-3-hydroxy-5-methylpiperidin-2-ones, Tetrahedron Lett., 2000, 41, 397.
Synthesis and enzyme-catalysed reductions of 2-oxo acids with oxygen containing side-chains, J. Chem Soc., Perkin Trans. 1, 2000, 901.
Syntheses of isotopically labelled L-amino acids with an asymmetric centre at C-3, J. Chem. Soc., Perkin Trans. 1, 2000, 3406.
Ugi four component condensations using aldehydes with an asymmetric centre at C-2, Tetrahedron Lett., 2000, 41, 8001.
Probes for the active sites of leucine dehydrogenase, Bioorg. Med. Chem. Lett., 1999, 9, 1941.
Chemoenzymatic synthesis of 4-amino-2-hydroxy acids: A comparison of mutant and wild-type oxidoreductases, J. Org. Chem., 1998, 63, 7764.