![]()
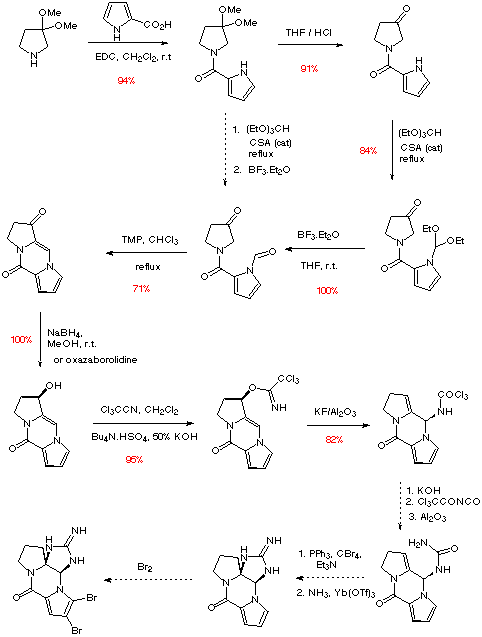
Scheme 5
As can be seen, the aldol / Mannich cyclisation in this case proceeds in good yield, the stability of the iminium species presumably helped by the presence of the aromatic ring. As in the synthesis of (-)-slaframine, it is hoped that enantioselective reduction of the enone with a chiral oxazaborolidine salt of borane will form the required chirality. Elaboration of this allylic alcohol to the corresponding allylic trichloroimidate sets up the key sigmatropic rearrangement. Hydrolysis of the amide formed on rearrangement, followed by acylation to the imide, and hydrolysis to the corresponding urea will then give a product similar to that used by Kishi in his synthesis of saxitoxin (Scheme 6).8
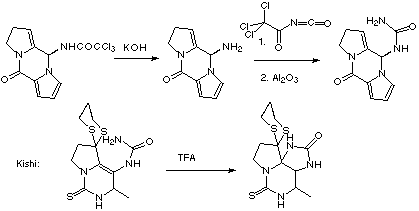
Scheme 6
In our case, the guanidine may be easier to incorporate at the acyclic stage, using the method of Isobe.
Finally, the lessons learned from the synthesis of dibromophakelin can be utilised in developing a strategy for the synthesis of saxitoxin.
This work was carried out during the course of my Ph.D. studies at the School of Chemistry, University of Bristol, under the supervision of Professor Timothy Gallagher.
Some of this work was published in Synlett 1996, 853. The results disclosed in this paper are represented on the net here.