School of Chemistry, University of Bristol, Bristol, BS8 1TS, United Kingdom
Abstract: Addition of a variety of simple and functionalised organozinc reagents (R2Zn or RZnX) to a structurally diverse range of glycals takes place in the presence of either BF3.OEt2 or TMSOTf and efficiently leads to C(1)-glycosides with selectivity for the alpha-isomer.
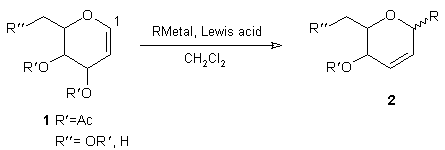
The electrophilic reactivity of glycals 1 towards carbon nucleophiles provides a versatile entry into C(1)-glycosides 2 via the carbon-Ferrier reaction.1,2 Of the organometallic nucleophiles (RMetal) available, zinc-based reagents (e.g. R2Zn, RZnX) are particularly attractive as vehicles for this transformation being readily generated and, more importantly, tolerant of a spectrum of functionality, both within the zinc reagent itself as well as in the electrophilic glycosyl component.3

The reaction of organozincs with glycals to give C-glycosides 2 has been reported, but this has only been achieved using a simple Reformatsky reagent.4 C-Aryl variants of 2 have also been prepared via ArZnX in the presence of Pd(0), but this is based on a stereospecific displacement of 1-O-acetyl-2,3-dideoxyhex-2-enopyranosides - the DELTA2-isomer of glycal 1.5
The more general aspects of this zinc-mediated approach to C-glycoside synthesis remain to be defined and in this paper we describe the reaction of alkyl organozinc nucleophiles towards a series of glycal derivatives. This process requires Lewis acid activation, but will permit the presence of acetate and benzyl O-protecting groups, as well as an interglycosidic linkage within the glycosyl electrophile, and flexibility within the organozinc component.
The results of this study are presented in two parts. Table 1 illustrates the scope of the chemistry available in terms of the glycal substrates that have been utilised with Et2Zn acting as a prototypical organozinc nucleophile. In Table 2, the ability to vary the functional groups associated with the organozinc nucleophile is displayed based on employing 3,4,6-tri-O-acetyl-DELTA-glucal as the electrophilic component.
The results shown in Table 1 show that good yields of C-glycoside products are available, but it is important to appreciate that the order of addition of reagents was crucial; exposure of the glycal to BF3.OEt2 prior to addition of Et2Zn resulted in poor yields. Usually only one equivalent of BF3.OEt2 was needed, except for substrates containing either O-benzyl units (entry 5, Table 1) or a high degree of oxygenation, as with hepta-O-acetylmaltal (entry 7, Table 1) where up to 5 equivalents of the Lewis acid were employed. In all cases, including the examples shown in Table 2, the alpha-C(1)-glycoside was the predominant product. The configuration at C(1) (alpha vs. beta) was based primarily on 13C NMR analysis in accordance with earlier studies; the chemical shift of C(5) is diagnostic and in the alpha-isomer is shielded relative to the corresponding beta-isomer.6 With the pentose-derived products (entries 1 and 2, Table 1), 13C NMR was less useful and, as a consequence, assignments in these two cases are viewed as tentative, but parallel the course of related C-glycosylation reactions.7
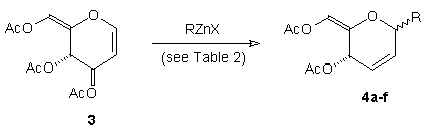
The ability to utilise zinc reagents that introduce useful functionality within the C-glycoside product was important to our longer term goals and variation in the structure of the organozinc reagent has been examined using 3,4,6-tri-O-acetyl-D-glucal 3. This led to C-glycosides 4 and the range of products available, together with yields and C(1)-isomer ratios, are presented in Table 2.
In the course of these studies two specific problems were encountered. The pivaloyl-based reagent (t-BuOCOCH2ZnI)8, which is equivalent to "·CH2OH", was less stable than the other alkyl zinc reagents used and C-glycoside 4e was obtained in only 29% yield. With the nitrile-containing reagent NCCH2CH2CH2ZnI,10 the zinc species was essentially insoluble (and unreactive) in CH2Cl2 and this reagent was also inert towards 3 in ether-based solvents. This difficulty was overcome by careful isolation of NCCH2CH2CH2ZnI from THF solution, followed by suspension in CH2Cl2 containing glucal 3 and BF3.Et2O, and the reaction mixture was then placed in a ultrasound bath. Not only was the carbon-Ferrier displacement observed using sonication, but the rate of this process to give 4f was also very substantially increased (10 min. vs. 3-8 h. for reactions of 3 with other alkyl organozinc reagents). In the other cases shown in Table 2, solubility and stability of the organozinc species was not a significant barrier and the successful incorporation of useful residues at C(1) illustrates an advantage of using organozinc reagents over a host of other metal-based nucleophiles.
The balance between reactivity and tolerance of functionality is an essential feature of this approach to C-glycoside synthesis. Glycals are relatively unreactive electrophiles and, by necessity, will often contain protecting groups that preclude the use of more nucleophilic, but acid-sensitive organometallic reagents. Zinc-based methodology offers a flexible and direct entry into functionalised C-glycosides and further development of this chemistry, including the use of sonication to enhance reactivity, is underway.
Experimental Procedures: General reaction conditions involved addition of the pre-formed organozinc reagent to a solution of the glycal in dry CH2Cl2 at -20°C followed by addition of the Lewis acid component. Et2Zn was used in hexane solution, but in cases where the zinc reagent was prepared in THF, this solvent was removed in vacuo (see below) prior to addition of CH2Cl2. The reaction mixtures were allowed to warm to r.t. over 30-60 min. (for reactions involving Et2Zn, Table 1) or 3-8 hr. (for most reagents used in Table 2) and the products were isolated after an aqueous work-up and chromatography. With NCCH2CH2CH2ZnI, once halogen-zinc exchange was complete, the THF solution of the zinc reagent was evaporated in vacuo (at 30-40°C and maintaining an atmosphere of argon when appropriate) to give a viscous grey liquid which was then taken up in CH2Cl2 and treated with glucal 3 and BF3.OEt2 at r.t. The mixture was then placed in a conventional laboratory ultrasonic bath for 10 minutes after which time glucal 3 had been consumed.11
Acknowledgement. We thank EPSRC (Grant No. GR/H92142) for financial support.
| Entry | Glycal | C-Glycoside | alpha:beta (% yield) |
|---|---|---|---|
| 1 |  |  | 24:1 80% |
| 2 |  |  | 12:1 53% |
| 3 |  |  | 1.5:1 71% |
| 4 |  |  | 3.2:1 95% |
| 5 |  |  | 4.1:1 87% |
| 6 |  |  | alpha only 73% |
| 7 |
Maltal (R = Ac) | 
| 2:1 81% |
| C-Glycoside 4 | Zinc Reagent Lewis acid | alpha:beta ratio % yield |
|---|---|---|
 4a | PhCH2ZnBr TMSOTf (1 equiv.) | 1.7:1 57% |
 4b | PhCH2CH2ZnI BF3.OEt2 (1 equiv.) | 7:1 53% |
 4c | Cl(CH2)4ZnI BF3.OEt2 (3 equiv.) | 9:1 63% |
 4d | EtO2C(CH2)3ZnI BF3.OEt2 (6 equiv.) | 5:1 87% |
 4e | t-BuOCOCH2ZnI BF3.OEt2 (4 equiv.) | 4:1 29% |
 4f | NC(CH2)3ZnI BF3.OEt2 (3 equiv.) | 5:1 63% (See text) |
| Organozinc Reagents for the Nucleophilic C-Glycosylation of Glycals. S. N. Thorn and T. Gallagher C-Glycosides, organozinc reagents, ultrasound | 
|