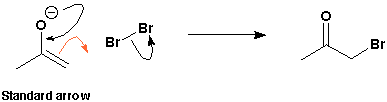
1999 © Paul Wyatt, University of Bristol
Consider a curly arrow which indicates the breaking of a bond and the forming of a new bond.
When an enolate reacts with an electrophile (such as, say, bromine) the curly arrow starts in the middle of the carbon-carbon bond which is breaking and ends in the middle of the new forming carbon-bromine bond. The product is an a-bromoketone. The curly arrow is a standard curly arrow. There is nothing wrong with this whatsoever.
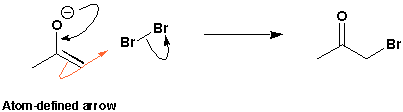
The same mechanism can be drawn with an atom-defined curly arrow. Notice that this also starts in the middle of the bond which is breaking and ends in the middle of the forming bond just like a standard curly arrow. But, it contains an extra piece of information about the mechanism. It loops through the atom to which the new bond is being formed. This type of arrow can sometimes help you to keep track in a mechanism and not lose atoms or bonds and avoids any ambiguity. Several chemists like to employ this kind of curly arrow occasionally and Dr Wyatt uses them extensively with enolates. Both types are acceptable in exams but if you do choose to use atom-defined curly arrow be sure to get them right.
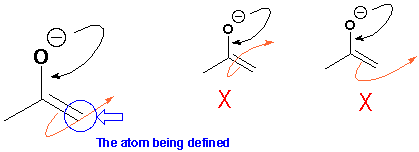
In the above situation it is fairly obvious which carbon bond is nucleophilic but this is not always the case. In the reaction below a double bond reacts with an electrophile. What is the result? Did the person who wrote this mean to form A or to form B?
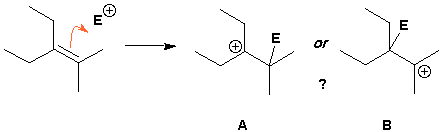
All ambiguity can be removed by using an atom-defined curly arrow.
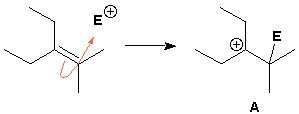
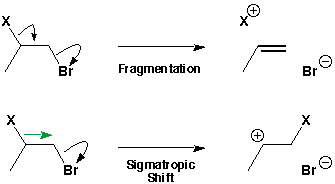
There are other curly arrow variations. The 'straight'curly arrow is recommended by Drs. Cox, Lloyd-Jones and Wyatt for migration (sigmatropic shift) reactions.