The Variation Principle:
If an arbitrary or approximate wavefunction
is used to calculate the energy of a system then the value obtained is always greater
than or equal to the exact ground state energy of the system.
This principle can be stated
mathematically as:
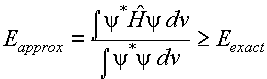 (1)
(1)
Another way of saying this is
The energy of an approximate wavefunction is never less than the true energy
The Variation principle is the key to
finding wavefunctions of molecules.