As an example,
consider HCl:
We can think of a wavefunction in
which the bond is formed between H and Cl by pairing an electron in the
hydrogen in the H 1s orbital with one
in the Cl 3pz
orbital.
This describes covalent bonding. Let’s call this wavefunction Ycov.
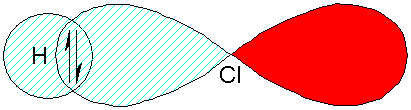 Ycov
Ycov
The energy of this wavefunction is ecov.
However, this wavefunction
doesn’t give the full picture of bonding in HCl: because the electrons are
paired, it doesn’t allow both electrons to be simultaneously on the chlorine
atom. It is only a partial
description. Chlorine is highly
electronegative, so it is quite likely that a full description would allow more
polar structures to play a part in the full description of HCl.
We can write down another wavefunction
which goes to the ionic extreme, and has both electrons on the chlorine atom,
i.e. representing the H+Cl– structure. In this wavefunction, Yion, both electrons are in the chlorine 3pz orbital.
Both of these wavefunctions, Ycov and Yion, give only a partial description
of the bonding in HCl, each representing one extreme. A better overall description - a better wavefunction- is given by
combining the two:
Y = ccovYcov + cionYion
The coefficients ccov and cion
are numbers which give the relative contribution of the ionic and covalent
forms.
The variation principle tells us that
the best wavefunction (of this sort) is the one with the lowest energy.
This means we can find the best
wavefunction by varying the coefficients until we find the wavefunction which
has the lowest energy - this is the variational method.
We might find, for example, that the best wavefunction at a particular internuclear distance is
y = 0.3 ycov + 0.95 yion
(indicating that the ionic form plays
a bigger role than the covalent form).
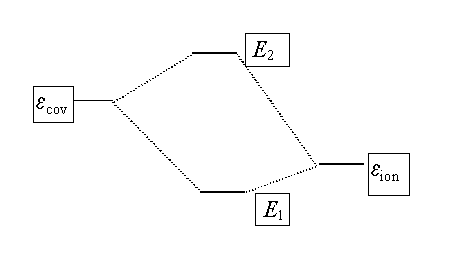
The energy of the better wavefunction resulting from a combination of the original wavefunctions is lower than the energies of either of the original wavefunctions.
The values of
the coefficients is found by minimizing the energy with respect to them - the variational
method.
The variational method is the most
important way of finding molecular wavefunctions.
Molecular Orbitals and the Linear
Combination of Atomic Orbitals (LCAO) Approximation