If we multiply out the secular determinant we
obtain:
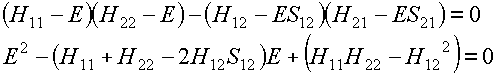 (16)
(16)
which you should now be able to recognise as a
quadratic equation for E. It has two solutions E1 and E2. These are the two molecular orbital
energies.
We now take one of these, E1 , and place it in the secular equations (eq.
14). These equations now have a
nontrivial solution and we find the ratio
c2/c1 by solving them. The absolute value of the coefficients c1
and c2 can only be found by using the normalization condition:
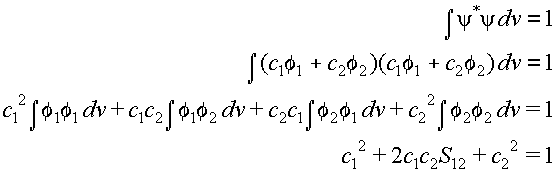 (17)
(17)
We now add an extra index to the coefficients c1
and c2 to show that they belong to molecular orbital no.
1. We obtain the coefficients for
molecular orbital No. 2 in the same way by substituting the second solution of
the secular equations, E2
, into the secular equations.
Molecular orbital no. 1
Molecular orbital energy: E1
Molecular orbital: ![]()
Molecular orbital no. 2
Molecular orbital energy: E2
Molecular orbital: ![]()