Returning to the example of HCl from lecture one, at
any fixed internuclear separation the best wavefunction is a mixture of two
wavefunctions, yion
and ycov, describing respectively an
ionic form of HCl (both electrons on the chlorine), and a covalent form (one
electron on each atom).
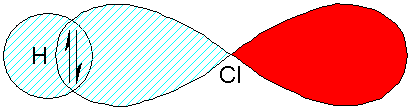 Ycov
Ycov
H+ Cl–
Yion
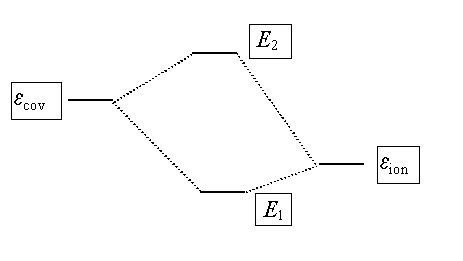
Y = cion Yion + ccov Ycov
The coefficients cion and ccov can be found by the variational method (see lecture
1).
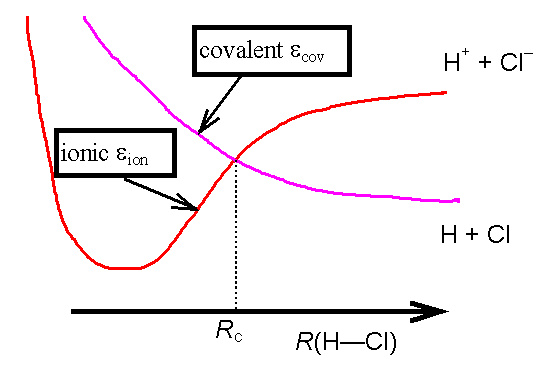
This is the situation at a particular fixed H--Cl
internuclear separation. At different
internuclear separations, the coefficients found by the variational method will
be different. For example, HCl
dissociates into neutral atoms, so ycov (which has one electron in
the Cl 3p orbital, and one in the H 1s orbital) is a better description at
large internuclear separations. At
short distances, though, the molecule is much more polar, with more tendency
for both electrons to be associated with the chlorine, so yion is a better
description. As the nuclear separation
changes the potential energy curves corresponding to the “ionic” and “covalent”
wavefunctions cross.
However, we get a better
description (a better wavefunction) by allowing the ionic and covalent wavefunctions to mix, giving two new ‘mixed’
states with energy E1 and E2.
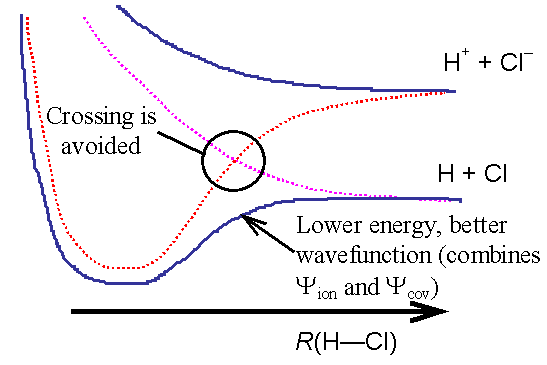
At every internuclear separation the original
energies split apart. The new energies
“E” are better in the sense that they
are closer to the exact correct results.
At points where the original energies (eion and ecov ) are very close (or cross)
the new better energies split apart and no longer cross. The point of closest approach is called an avoided crossing point.