Finding the p molecular orbitals of ethane using the LCAO approximation
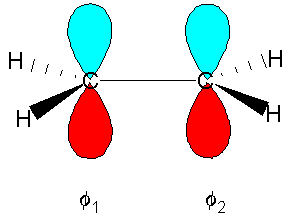
The expression for the
energy can be written as:
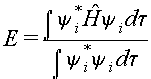
where the integrals are over
all space. The energy calculated in
this manner will depend on the coefficients ci1,
and ci2,
The variational principle states
that if an approximate wavefunction (such as this) is used to calculate the
energy, the value obtained for the energy is always greater than or equal to
the exact ground state energy of the system, i.e.
E ³ Eexact
So the next stage in this case is to minimize the energy with
respect to the cip,, i.e, ci1 and ci2. This minimized energy will still be above
the true ground state energy. The
wavefunction with the values of ci1
and ci2 that
give the minimum energy is the best wavefunction it is possible to obtain using
just a simple combination of two atomic orbitals. It gives the energy closest to the exact ground state energy.
As we have seen in the previous lecture this leads to secular equations and the secular determinant:
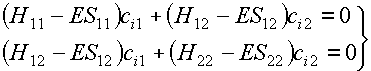
We can write them in matrix form:
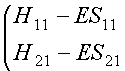
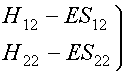
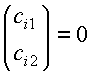 (2)
(2)
The trivial solution of these is ci1 = ci2 =
0. (Why is this of no interest?)
Next: the secular determinant and the
energies of the molecular orbitals of ethene