Other examples of aromatic molecules
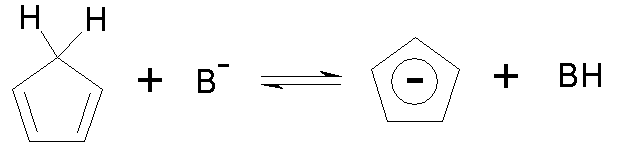
Cyclopentadienyl anion (C5H5–)
· 6 electrons in p system, aromatic with 4n+2 (n=1) p electrons.
· Delocalization
energy =2.47b.
· So predict
cyclopentadiene to lose a proton relatively easily.
Experimentally:
pKa
of cyclopentadiene =16 (not very different from water!)
_____________________________________________
In contrast, cyclobutadiene (C4H4)
is extremely difficult to make in the laboratory.
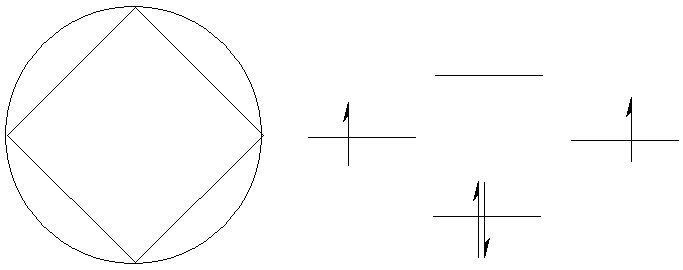
p-electron
energy
= 2´(a+2b) +2a
= 4a +4b
Delocalization energy
= 4a +4b – (2´(2a+2b))
= 0
Cyclobutadiene is described as ‘anti-aromatic’