The p molecular orbitals of 1,3-butadiene
What do the
orbitals look like?
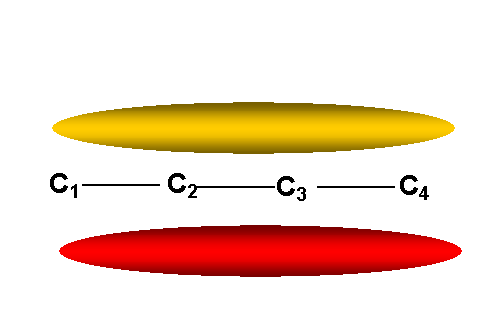
y1 -- lowest
energy p M.O.
-- no nodes other than in
molecular plane:
______________________________________________________________
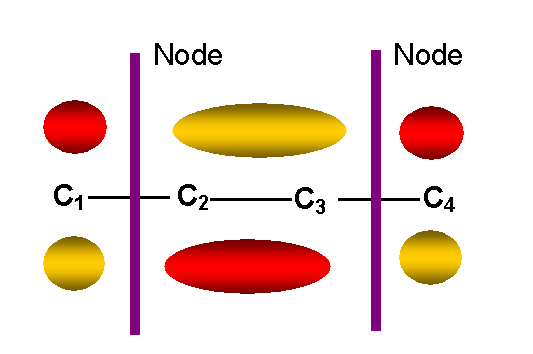
y2 -- second
lowest energy p MO
-- one node other than in molecular plane:
______________________________________________________________
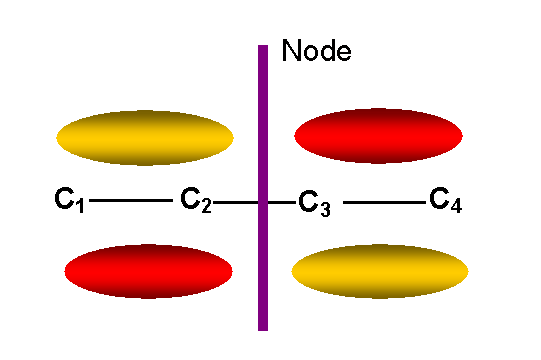
y3 -- first
unoccupied antibonding p MO
-- two nodes other than in
molecular plane:
______________________________________________________________
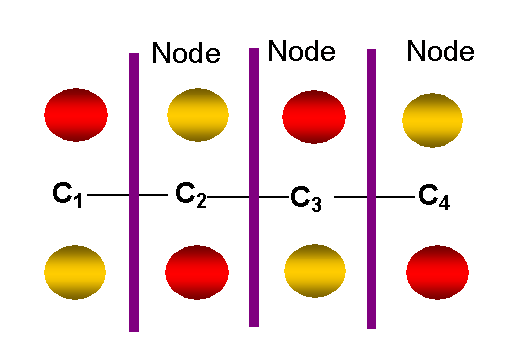
y4 -- highest
antibonding p MO
-- 3 nodes other than in molecular plane: