Cyclic Conjugated Polyenes
The ‘Circle’ Method; Benzene and
Aromaticity
See Murrell, Kettle and Tedder “Valence
Theory” page 268,
Carroll ‘Perspectives on Structure and
Mechanism in Organic Chemistry’ pgs. 205-208
It is easy to write down the secular equations and secular determinant for benzene.
We number the carbon atoms as below:
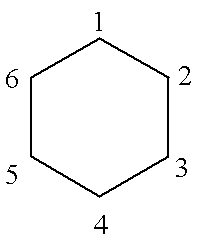
Consider atom No. 1. It is next to atoms 6 and 2; so the corresponding secular equation is:
xc1 + c2 + 0´c3 + 0´c4 + 0´c5+ c6 = 0
or
xc1 + c2 + 0 + 0 + 0 + c6 = 0
· Each secular
equation involves just 3 non-zero terms.
· All the six
secular equations have the same structure, and they can be obtained from each
other by permuting the indices (i.e. by just adding one to the indices of the
preceding equation.
· The equations
can be solved analytically making use of trigonometric functions.
· These
trigonometric solutions may in turn, rigorously, be represented by a simple
geometric construction- this is the ‘Circle method’