Cycloaddition reactions
Why is is that cis-1,3-butadiene and ethene react to form cyclohexene when heated:
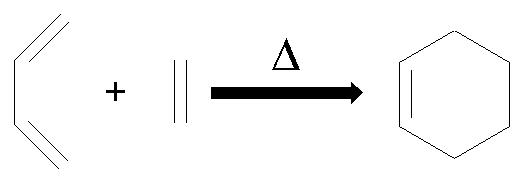
(Diels-Alder reaction, 4 +2 cycloaddition)
but the
analogous cycloaddition of two ethene molecules does not work?
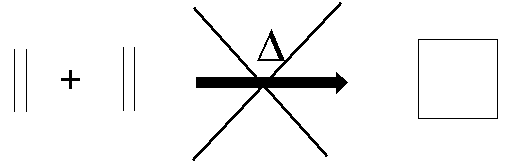
(2+2 cycloaddition, calculated to be exothermic by 75 kJ/mol)
We can explain and understand this
through the principles of conservation of orbital symmetry.