Cycloaddition of 2 ethenes to
form cyclobutane (ethene dimerization)
We consider the reactants (2 ethene
molecules) coming together so that their p orbitals
overlap.† This is the pathway for the
reaction:
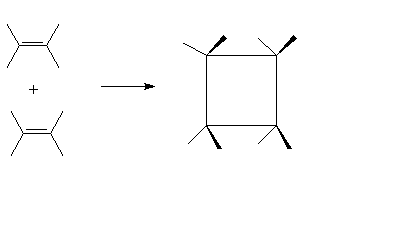
i.e.
∑
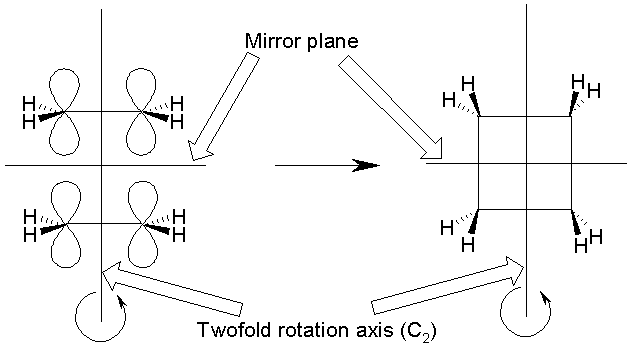
Two symmetry ELEMENTS (a two-fold rotation axis, C2, †and a mirror plane) are conserved throughout
the reaction Ė
∑
They are present in the reactants (two ethenes) as they approach
one another, and also in the products (and in the transition state of the
reaction).
∑
These symmetry elements are shown above for the reactants and
the products.†
∑
There are other symmetry elements, but we will consider only the
minimum number we need to analyse the reaction.†
∑
We must now classify the symmetries of the orbitals of the
reactants and the products with respect to these symmetry elements.†
We must
consider the combinations of the orbitals for their symmetry properties
(e.g. the MOs of one ethene alone donít have these symmetry elements).†
The combined MOs must be either symmetric (S) or antisymmetric (A) for each symmetry element.†